Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I'm having some issues with this chart, the graph and the question asked post lab. would you please help me solve all these? thank you!
I'm having some issues with this chart, the graph and the question asked post lab. would you please help me solve all these? thank you! 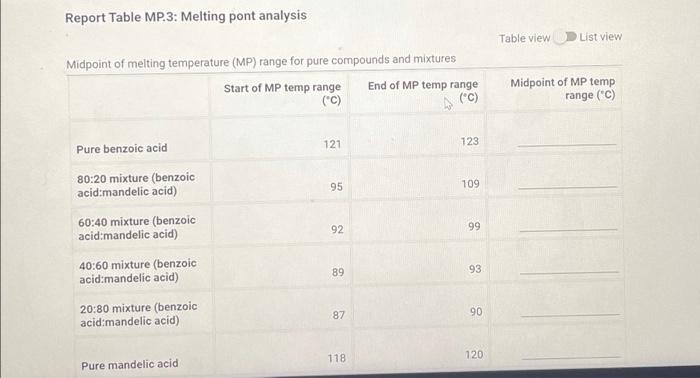
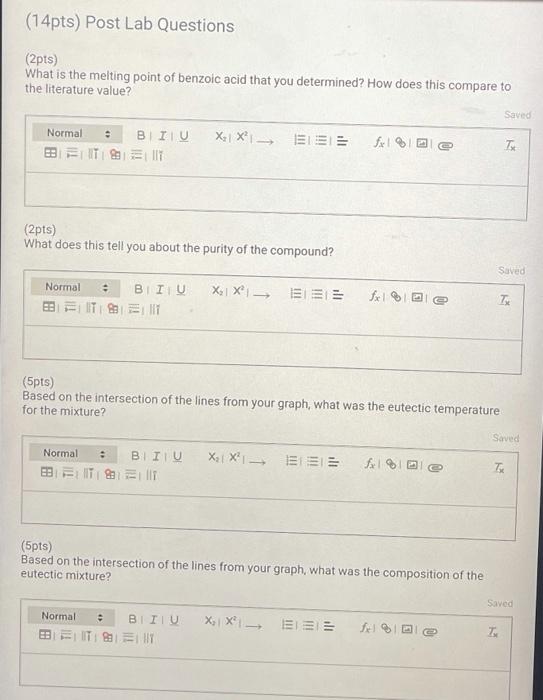
Report Table MP.3: Melting pont analysis Table view List view Midpoint of melting temperature (MP) range for pure compounds and mixtures Start of MP temp range End of MP temp range (C) Midpoint of MP temp range (C) (c) 121 123 Pure benzoic acid 95 109 80:20 mixture (benzoic acid mandelic acid) 60:40 mixture (benzoic acid mandelic acid) 40:60 mixture (benzoic acid mandelic acid) 92 99 89 93 20:80 mixture (benzoic acid mandelic acid) 87 90 118 120 Pure mandelic acid (4pts) Graph your results of the pure compound and mixture melting points. For each point, use the temperature in the middle of your melting point range. After plotting all the points, draw two best fit straight lines to connect at the eutectic composition Upload your graph or Excel file here. Browse your files to upload or Drag and Drop Max attachments: 10 Max Size: 20.00MB each (14pts) Post Lab Questions (2pts) What is the melting point of benzoic acid that you determined? How does this compare to the literature value? Saved Normal BIU BITIT X1 X fx 1 T (2pts) What does this tell you about the purity of the compound? Saved Normal . BII U BET 1 XX 1913 e Tu (5pts) Based on the intersection of the lines from your graph, what was the eutectic temperature for the mixture? Saved Normal . BII U 3 IT allt XXI == fx % Te (5pts) Based on the intersection of the lines from your graph, what was the composition of the eutectic mixture? Saved Normal BITU X, XI f BEIT UT 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


