Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In a particular gravimetric analysis, the precipitate of barium sulfate was weighed before it was completely dried. The likely Impact of this error on the

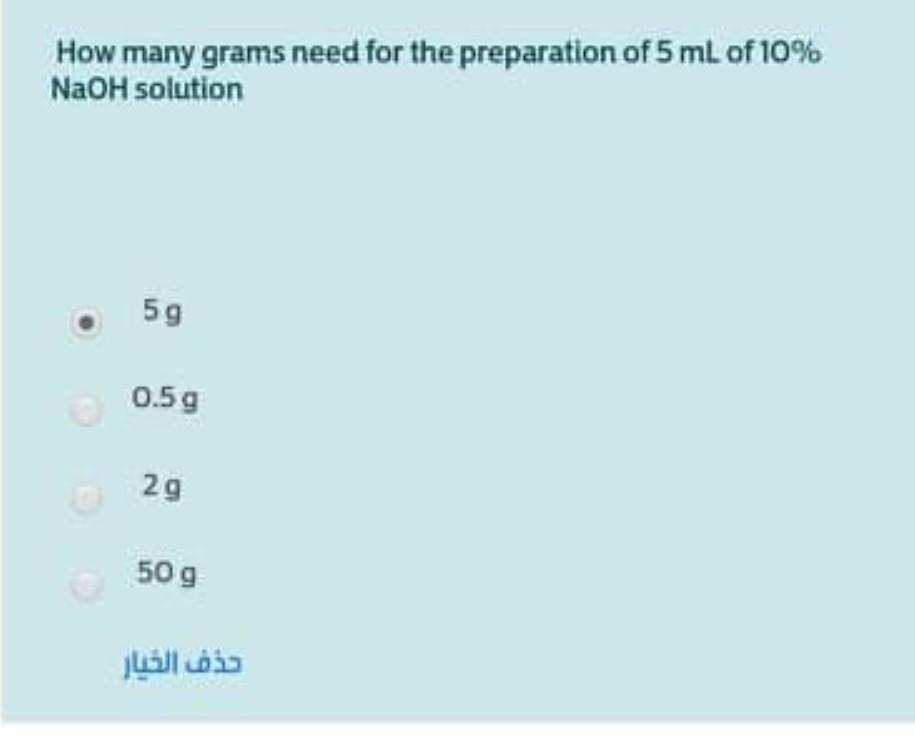



In a particular gravimetric analysis, the precipitate of barium sulfate was weighed before it was completely dried. The likely Impact of this error on the calculated result is... A lower result than the correct value. Minimal, because the mass of water is likely to be very low. Minimal, because the calculation assumes a hydrated sample is produced. A higher result than the correct value. How many grams need for the preparation of 5 mL of 10% NaOH solution 59 0.5g 2g 50 g What volume of 0.100 M HCL is required to neutralize 50.0 mL of a 0.150 M solution of Na2CO3? 150.ml 125 ml 75.0 ml 50.0 mL A 0.6745 gram sample of KHP reacts with 41.75 mL of NaOH solution for complete neutralization. What is the molarity of the KOH solution? (MW of KHP = 204 g/mol) 0.079 M 0.099 M 0.158 M 0.139 M The best procedure to use when filtering an aqueous solution to collect a precipitate is to... Use large volumes of water to ensure that all soluble matter Is separated from the precipitate. Not wash the precipitate at all. Warm the solution before filtering. Wash the precipitate with a minimal amount of water
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


