Answered step by step
Verified Expert Solution
Question
1 Approved Answer
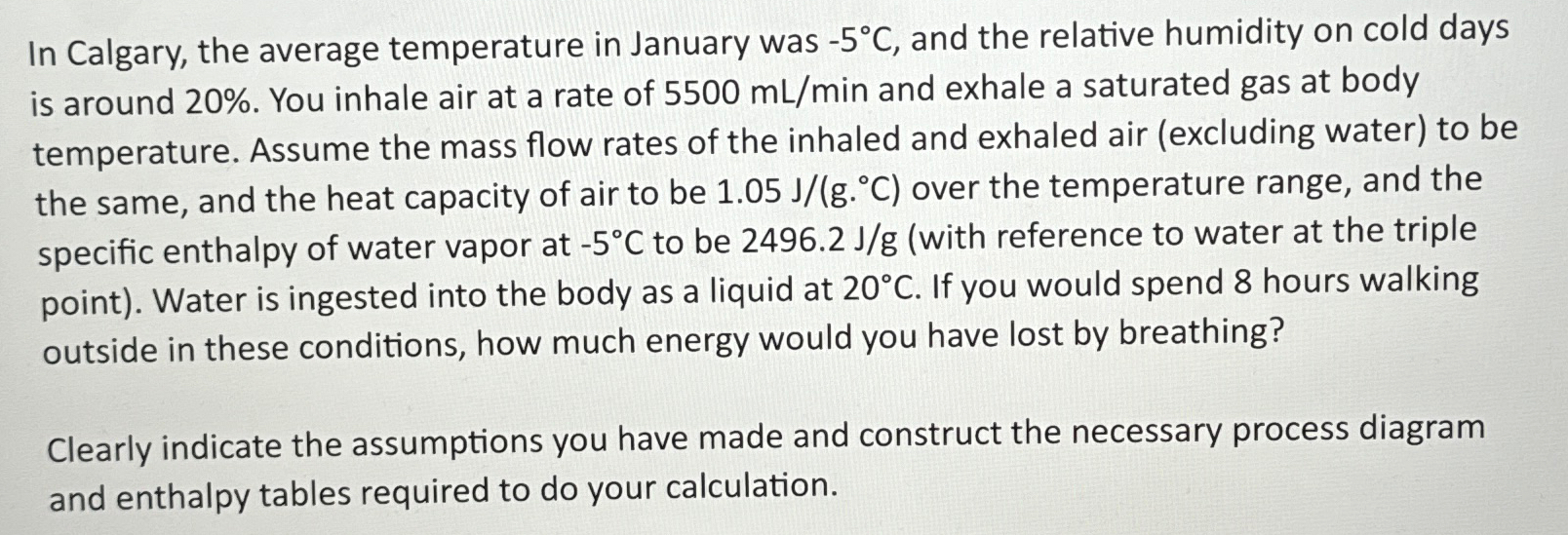
In Calgary, the average temperature in January was - 5 C , and the relative humidity on cold days is around 2 0 % .
In Calgary, the average temperature in January was and the relative humidity on cold days is around You inhale air at a rate of and exhale a saturated gas at body temperature. Assume the mass flow rates of the inhaled and exhaled air excluding water to be the same, and the heat capacity of air to be over the temperature range, and the specific enthalpy of water vapor at to be with reference to water at the triple point Water is ingested into the body as a liquid at If you would spend hours walking outside in these conditions, how much energy would you have lost by breathing?
Clearly indicate the assumptions you have made and construct the necessary process diagram and enthalpy tables required to do your calculation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


