Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In class, we argued that to describe a mixture with nc components in VLE, we need 2n, thermodynamic variables: T, P, ne - 1
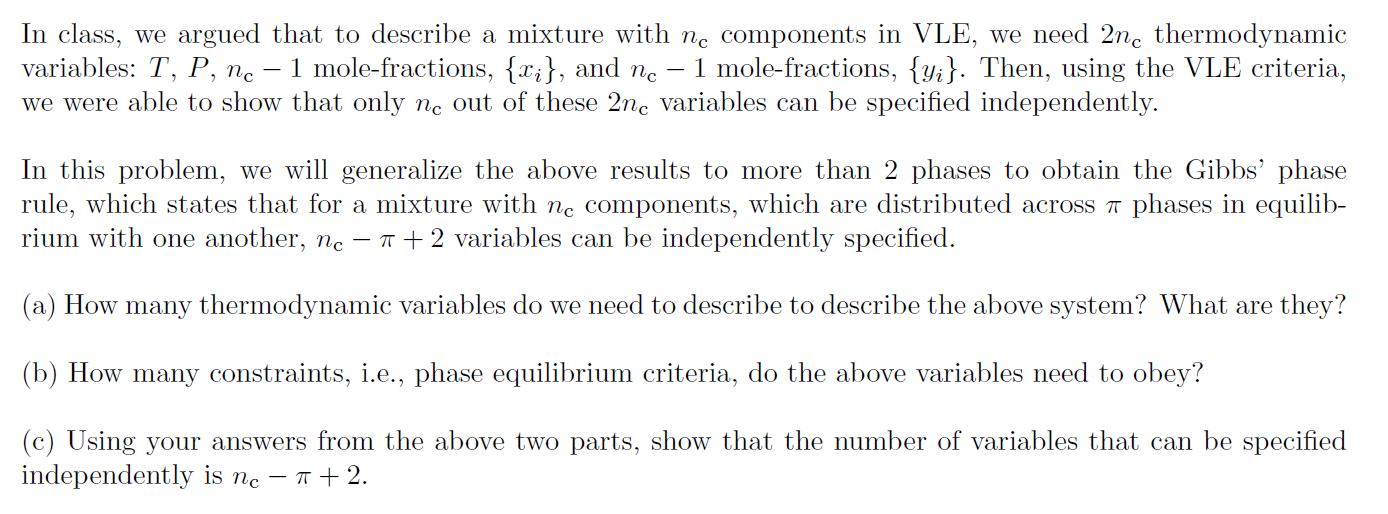
In class, we argued that to describe a mixture with nc components in VLE, we need 2n, thermodynamic variables: T, P, ne - 1 mole-fractions, {x}, and ne 1 mole-fractions, {y}. Then, using the VLE criteria, we were able to show that only ne out of these 2nc variables can be specified independently. In this problem, we will generalize the above results to more than 2 phases to obtain the Gibbs' phase rule, which states that for a mixture with nc components, which are distributed across phases in equilib- rium with one another, nc - + 2 variables can be independently specified. (a) How many thermodynamic variables do we need to describe to describe the above system? What are they? (b) How many constraints, i.e., phase equilibrium criteria, do the above variables need to obey? (c) Using your answers from the above two parts, show that the number of variables that can be specified independently is nc - +2.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


