Answered step by step
Verified Expert Solution
Question
1 Approved Answer
in lab , we conducted the synthesis of isoamy acetate by using the reagents below , im currently missing the theoretical yield of each ,
in lab , we conducted the synthesis of isoamy acetate by using the reagents below , im currently missing the theoretical yield of each , as well as the molar ratio , could you help me find them . the weight I got from the actual product synthesized was 17.004 g. 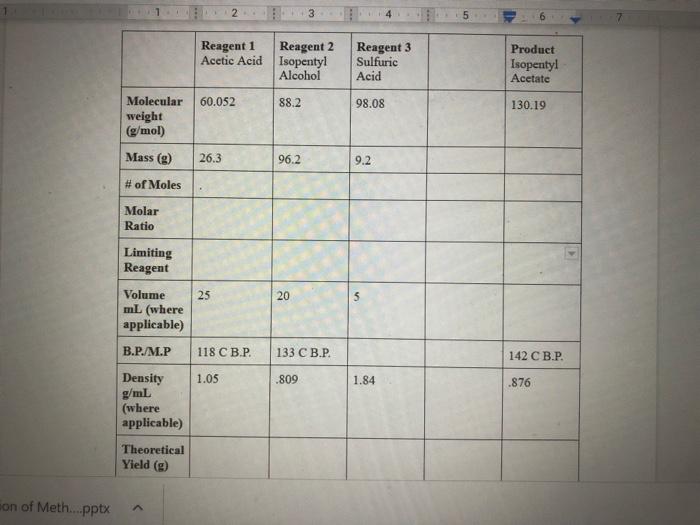
11 2 3 14 Reagent 1 Reagent 2 Acetic Acid Isopentyl Alcohol Reagent 3 Sulfuric Acid Product Isopentyl Acetate 60.052 88.2 98.08 130.19 Molecular weight (g/mol) Mass (8) 26.3 96.2 9.2 # of Moles Molar Ratio Limiting Reagent 25 20 5 Volume mL (where applicable) B.P./M.P 118 C B.P. 133 .. 142 C B.P. Density 1.05 .809 1.84 .876 g/mL (where applicable) Theoretical Yield (g) son of Meth....pptx 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


