Answered step by step
Verified Expert Solution
Question
1 Approved Answer
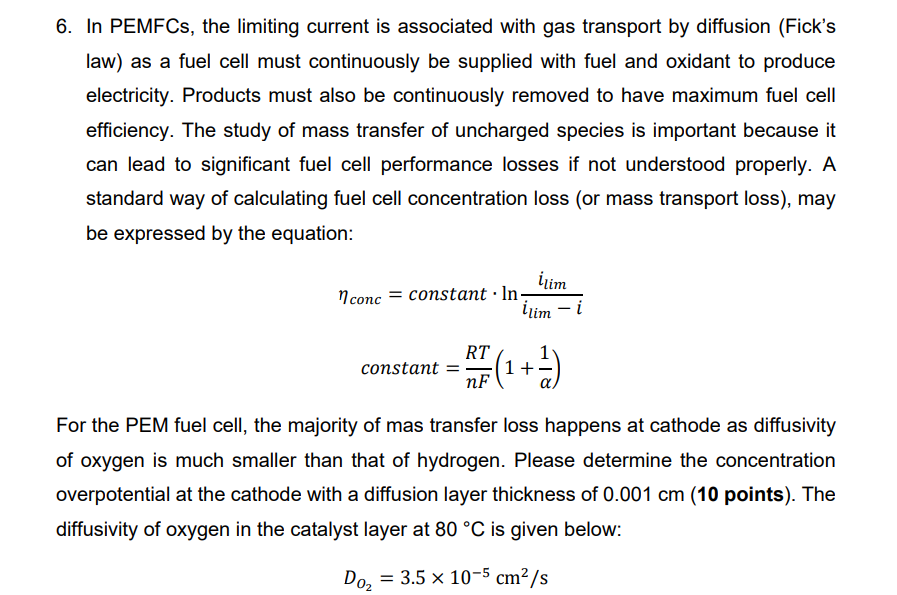
In PEMFCs, the limiting current is associated with gas transport by diffusion ( Fick s law ) as a fuel cell must continuously be supplied
In PEMFCs, the limiting current is associated with gas transport by diffusion Ficks law as a fuel cell must continuously be supplied with fuel and oxidant to produce electricity. Products must also be continuously removed to have maximum fuel cell efficiency. The study of mass transfer of uncharged species is important because it can lead to significant fuel cell performance losses if not understood properly. A standard way of calculating fuel cell concentration loss or mass transport loss may be expressed by the equation:
eta concconstant.lnilimilim
constantRT alpha nF
For the PEM fuel cell, the majority of mas transfer loss happens at cathode as diffusivity of oxygen is much smaller than that of hydrogen. Please determine the concentration overpotential at the cathode with a diffusion layer thickness of cm The diffusivity of oxygen in the catalyst layer at deg C is given below:
Dotimes cmsIn PEMFCs, the limiting current is associated with gas transport by diffusion Ficks
law as a fuel cell must continuously be supplied with fuel and oxidant to produce
electricity. Products must also be continuously removed to have maximum fuel cell
efficiency. The study of mass transfer of uncharged species is important because it
can lead to significant fuel cell performance losses if not understood properly. A
standard way of calculating fuel cell concentration loss or mass transport loss may
be expressed by the equation:
constant
constant
For the PEM fuel cell, the majority of mas transfer loss happens at cathode as diffusivity
of oxygen is much smaller than that of hydrogen. Please determine the concentration
overpotential at the cathode with a diffusion layer thickness of points The
diffusivity of oxygen in the catalyst layer at is given below:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


