Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In relation to the ethylene production via dehydrogenation of ethane reaction, the final goal of this project is to design a reactor system to produce
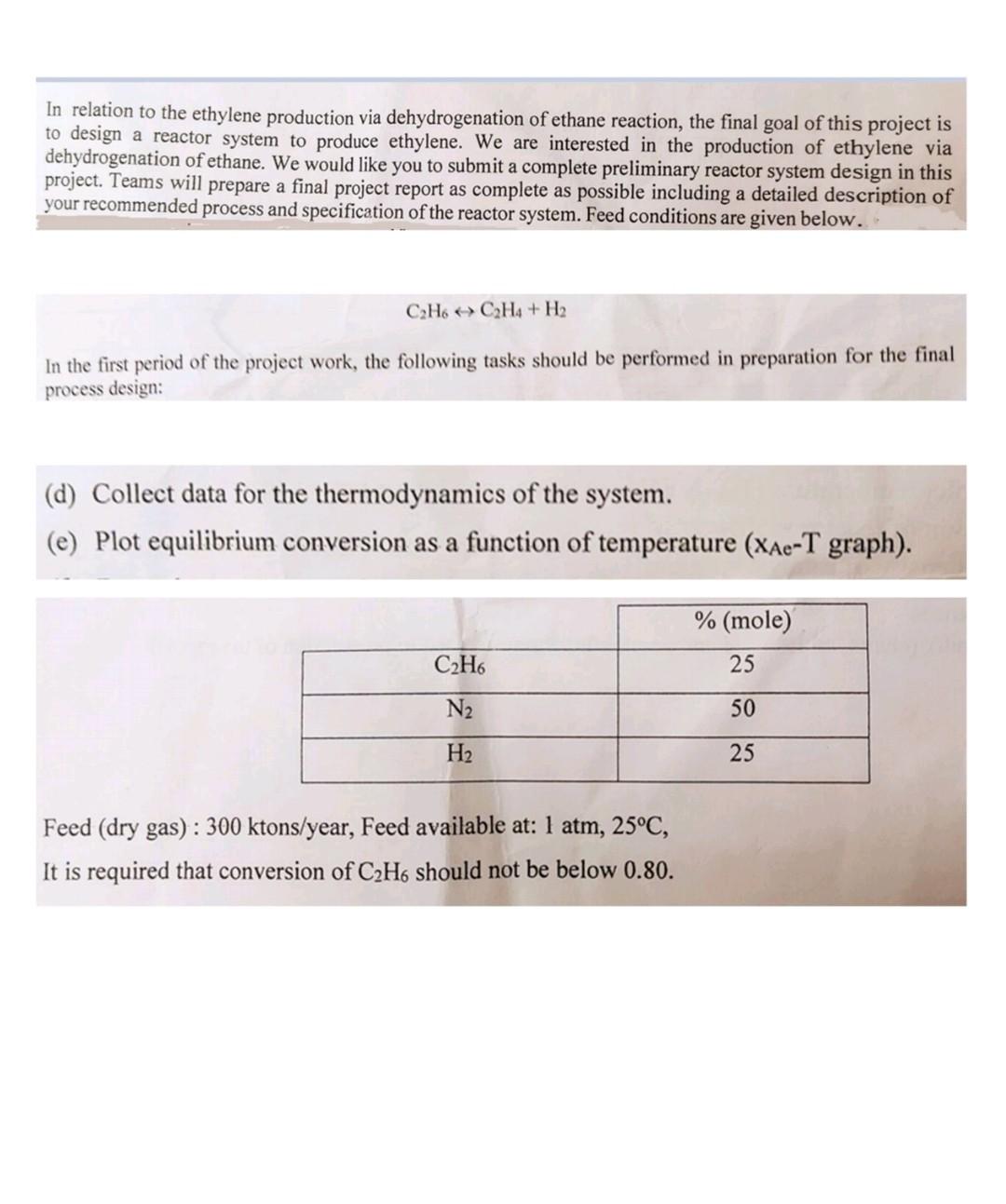
In relation to the ethylene production via dehydrogenation of ethane reaction, the final goal of this project is to design a reactor system to produce ethylene. We are interested in the production of ethylene via dehydrogenation of ethane. We would like you to submit a complete preliminary reactor system design in this project. Teams will prepare a final project report as complete as possible including a detailed description of your recommended process and specification of the reactor system. Feed conditions are given below.. C2H6 C2H4 + H2 In the first period of the project work, the following tasks should be performed in preparation for the final process design: (d) Collect data for the thermodynamics of the system. (e) Plot equilibrium conversion as a function of temperature (Xae-T graph). % (mole) 25 C2H6 N2 50 H2 25 Feed (dry gas) : 300 ktons/year, Feed available at: 1 atm, 25C, It is required that conversion of C2H6 should not be below 0.80
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


