Answered step by step
Verified Expert Solution
Question
1 Approved Answer
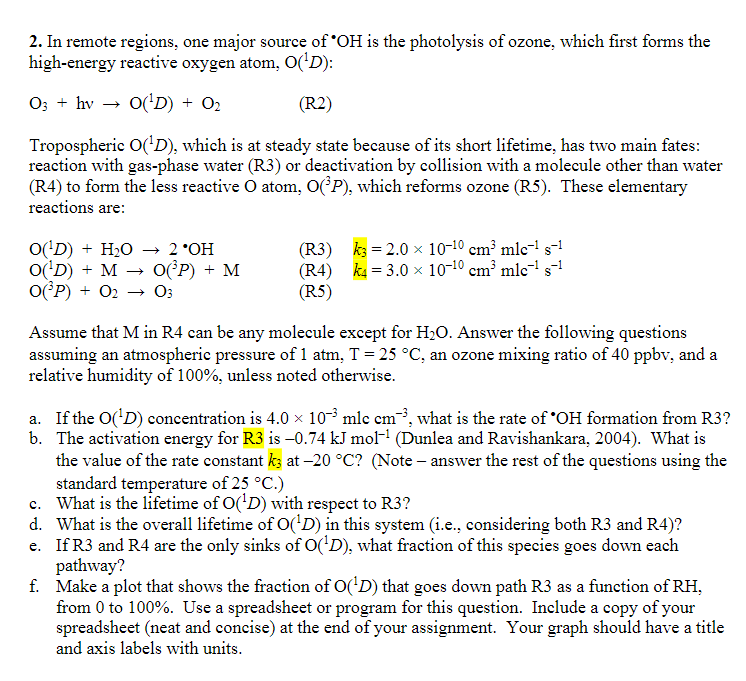
In remote regions, one major source of ? * O H is the photolysis of ozone, which first forms the high - energy reactive oxygen
In remote regions, one major source of is the photolysis of ozone, which first forms the
highenergy reactive oxygen atom, :
Tropospheric which is at steady state because of its short lifetime, has two main fates:
reaction with gasphase water or deactivation by collision with a molecule other than water
R to form the less reactive atom, which reforms ozone R These elementary
reactions are:
Assume that in can be any molecule except for Answer the following questions
assuming an atmospheric pressure of atm, an ozone mixing ratio of and a
relative humidity of unless noted otherwise.
a If the concentration is what is the rate of formation from
b The activation energy for is Dunlea and Ravishankara, What is
the value of the rate constant at Note answer the rest of the questions using the
standard temperature of
c What is the lifetime of with respect to
d What is the overall lifetime of in this system ie considering both R and R
e If and are the only sinks of what fraction of this species goes down each
pathway?
f Make a plot that shows the fraction of that goes down path as a function of
from to Use a spreadsheet or program for this question. Include a copy of your
spreadsheet neat and concise at the end of your assignment. Your graph should have a title
and axis labels with units.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


