Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the answer, you can see how it is saying that it is 1039 Kelvin. We have 1064 C and 25 degree C. We would

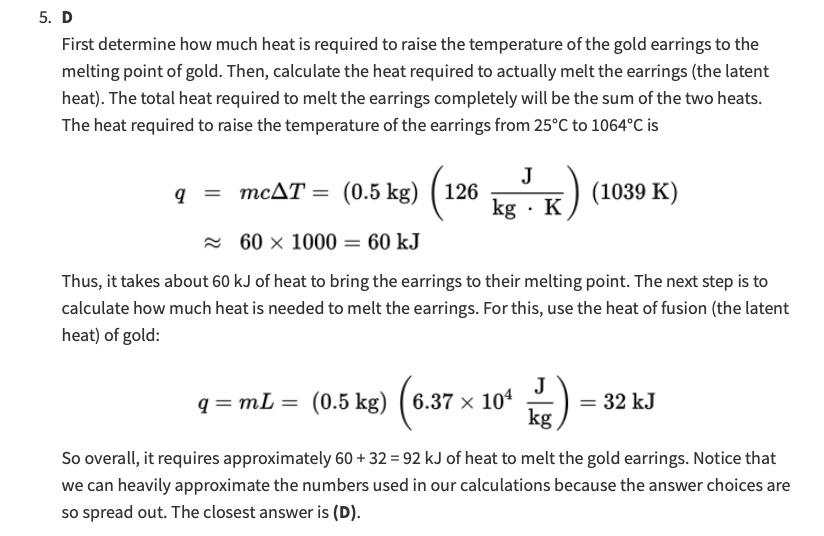
In the answer, you can see how it is saying that it is 1039 Kelvin. We have 1064 C and 25 degree C. We would subtract them right for final - initial and then we would get 1039 C. But to convert, wouldnt we have to add 273 to make it into kelvin which would make it 1312 K?

This is the work that I did. Please let me know if there is anything wrong here.
5. How much heat is required to completely melt a pair of gold earrings weighing 500g, given that their initial temperature is 25C ? (The melting point of gold is 1064C, its heat of fusion is 6.37104kgJ, and its specific heat is 126kgKJ.) (A) 15kJ (B) 32kJ (C) 66kJ (D) 97kJ D First determine how much heat is required to raise the temperature of the gold earrings to the melting point of gold. Then, calculate the heat required to actually melt the earrings (the latent heat). The total heat required to melt the earrings completely will be the sum of the two heats. The heat required to raise the temperature of the earrings from 25C to 1064C is q=mcT=(0.5kg)(126kgKJ)(1039K)601000=60kJ Thus, it takes about 60kJ of heat to bring the earrings to their melting point. The next step is to calculate how much heat is needed to melt the earrings. For this, use the heat of fusion (the latent heat) of gold: q=mL=(0.5kg)(6.37104kgJ)=32kJ So overall, it requires approximately 60+32=92kJ of heat to melt the gold earrings. Notice that we can heavily approximate the numbers used in our calculations because the answer choices are so spread out. The closest answer is (D). So, for this pootlew, you hare to find ont how anch heat is needet to reise the tempenture of the gold earips to mettig point. To do that, we use the formula q=mAT m=100ghesfobeconvertedtokgkg0g500g/kg=05kgc=126kgkJ=(0.5kg)(126kgkJ)(13/2k)=82656JkJ so, foom this we find that it tabes 82kJ of heat to bing it to its melting point. Now the next step is to calculeto how mun heat is nebel to melt the eariff To find ont how much heat ir needed to melt the eangs, we we the hets frovion which gives us the latent heat of sold. L113.85kJ82kJ+31.85kJq=mL=(0,5kg)(6.37104kgJ)=(0,5kg)(6.37104kgJ)=31850JkJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


