Answered step by step
Verified Expert Solution
Question
1 Approved Answer
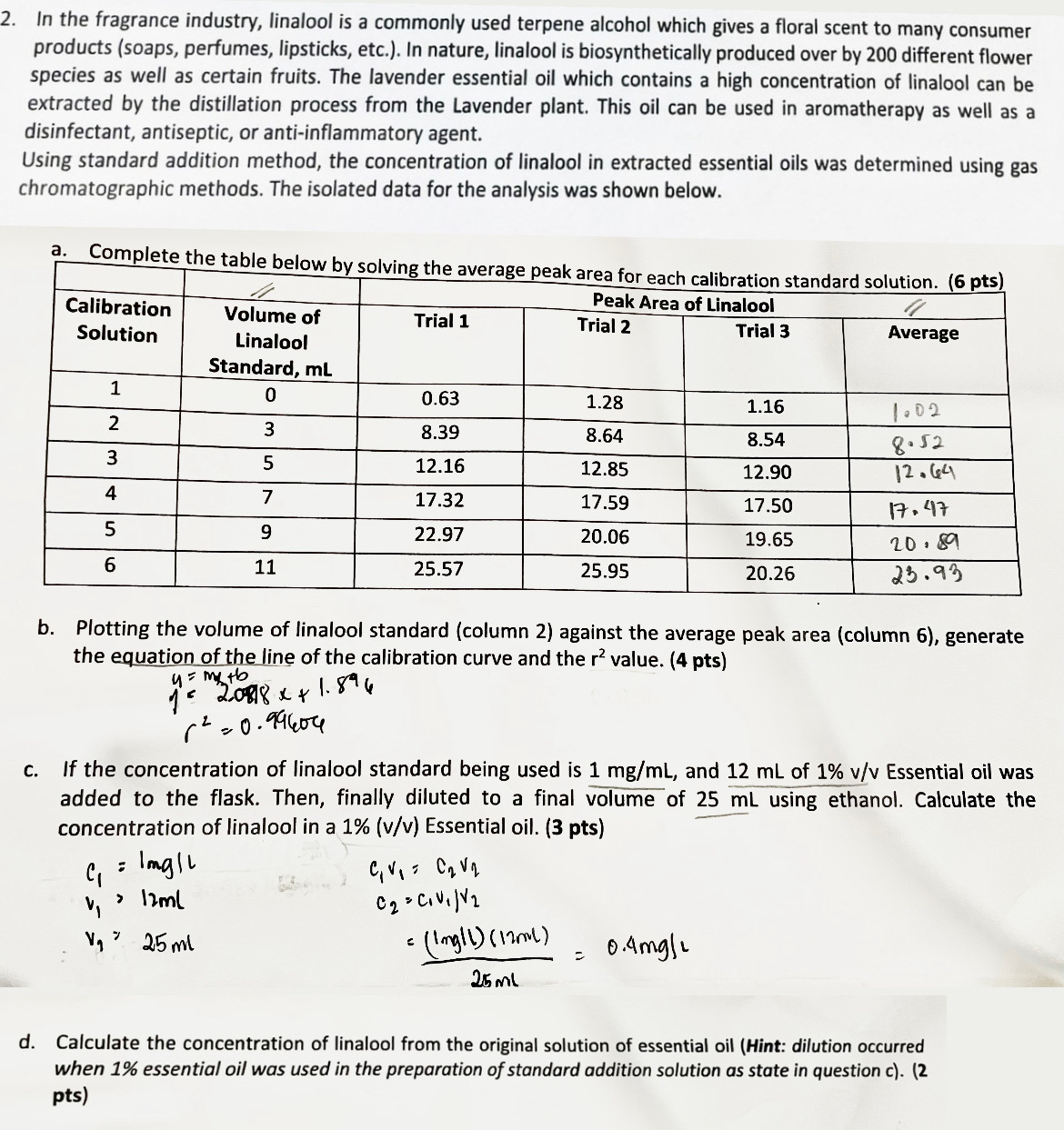
In the fragrance industry, linalool is a commonly used terpene alcohol which gives a floral scent to many consumer products ( soaps , perfumes, lipsticks,
In the fragrance industry, linalool is a commonly used terpene alcohol which gives a floral scent to many consumer products soaps perfumes, lipsticks, etc. In nature, linalool is biosynthetically produced over by different flower species as well as certain fruits. The lavender essential oil which contains a high concentration of linalool can be extracted by the distillation process from the Lavender plant. This oil can be used in aromatherapy as well as a disinfectant, antiseptic, or antiinflammatory agent.
Using standard addition method, the concentration of linalool in extracted essential oils was determined using gas chromatographic methods. The isolated data for the analysis was shown below.
a Complete the table below by solving the average peak area for each calibration standard solution. pts
b Plotting the volume of linalool standard column against the average peak area column generate the equation of the line of the calibration curve and the value. pts
c If the concentration of linalool standard being used is and of Essential oil was added to the flask. Then, finally diluted to a final volume of using ethanol. Calculate the concentration of linalool in a Essential oil. pts
d Calculate the concentration of linalool from the original solution of essential oil Hint: dilution occurred when essential oil was used in the preparation of standard addition solution as state in question c pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


