Question
In the process, 535 lb/h of fresh Ca(C2H3O2)2 and enough H2SO4 to be in 10.0% excess for converting the fresh Ca(C2H3O2)2 is fed into the
In the process, 535 lb/h of fresh Ca(C2H3O2)2 and enough H2SO4 to be in 10.0% excess for converting the fresh Ca(C2H3O2)2 is fed into the reactor. The single pass conversion of Ca(C2H3O2)2 in the reactor is 90.0%. Determine the amount of Ca(C2H3O2)2 recycled and the production rate of C2H4O2 by performing the given steps.
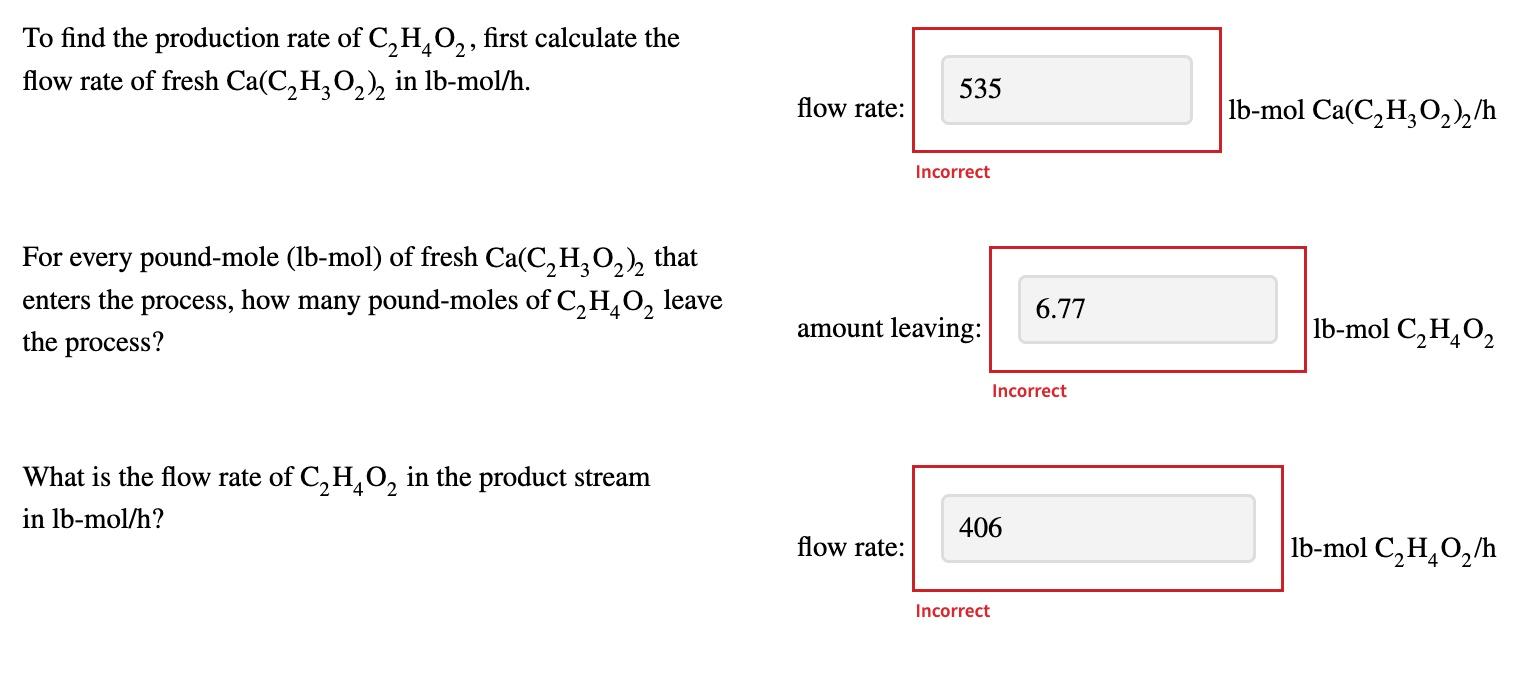
To find the production rate of C2H4O2,C2H4O2, first calculate the flow rate of fresh Ca(C2H3O2)2Ca(C2H3O2)2 in lbmol/h. ________
For every poundmole (lbmol) of fresh Ca(C2H3O2)2Ca(C2H3O2)2 that enters the process, how many poundmoles of C2H4O2C2H4O2 leave the process?_________
What is the flow rate of C2H4O2C2H4O2 in the product stream in lbmol/h? ____________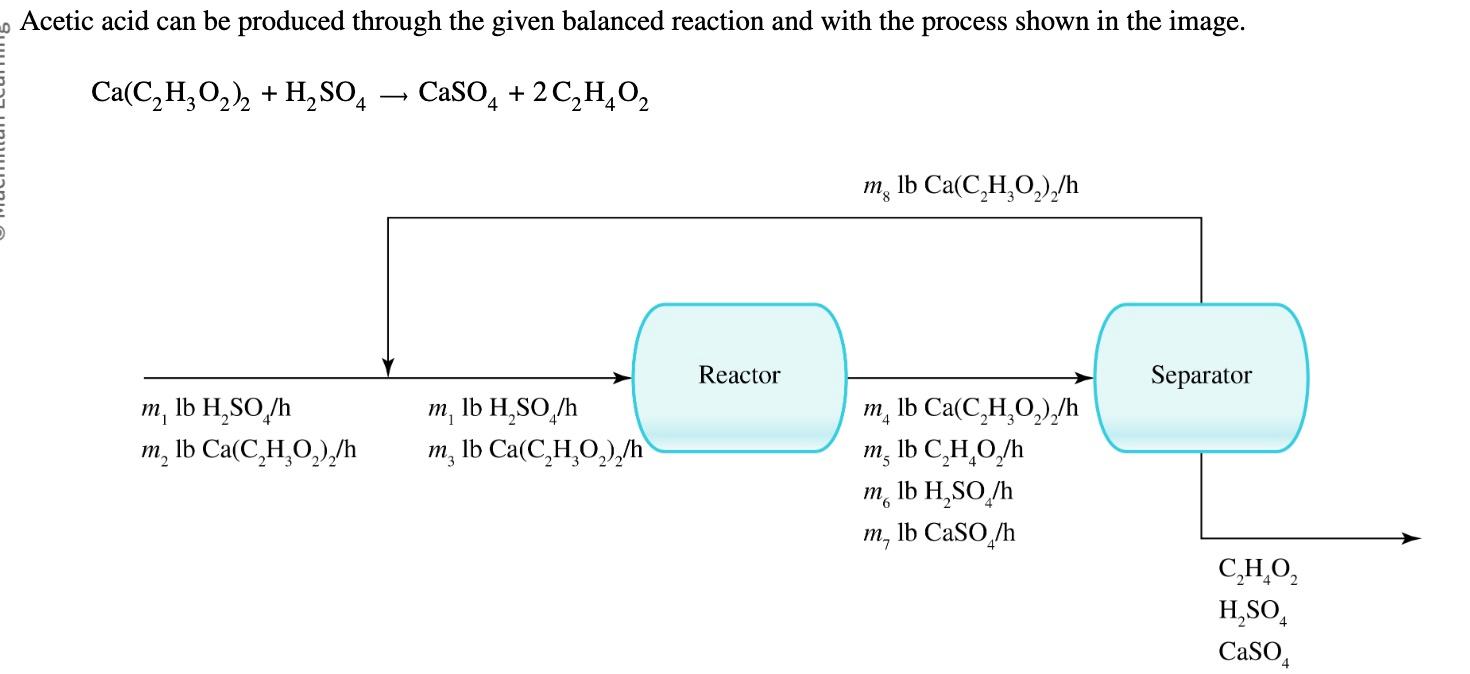
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


