Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Internal Combustion Engines COMBUSTION Ex6: The coal supplied to a boiler has the following analysis carbon 84 percent, hydrogen 6 percent and reminder ash. When
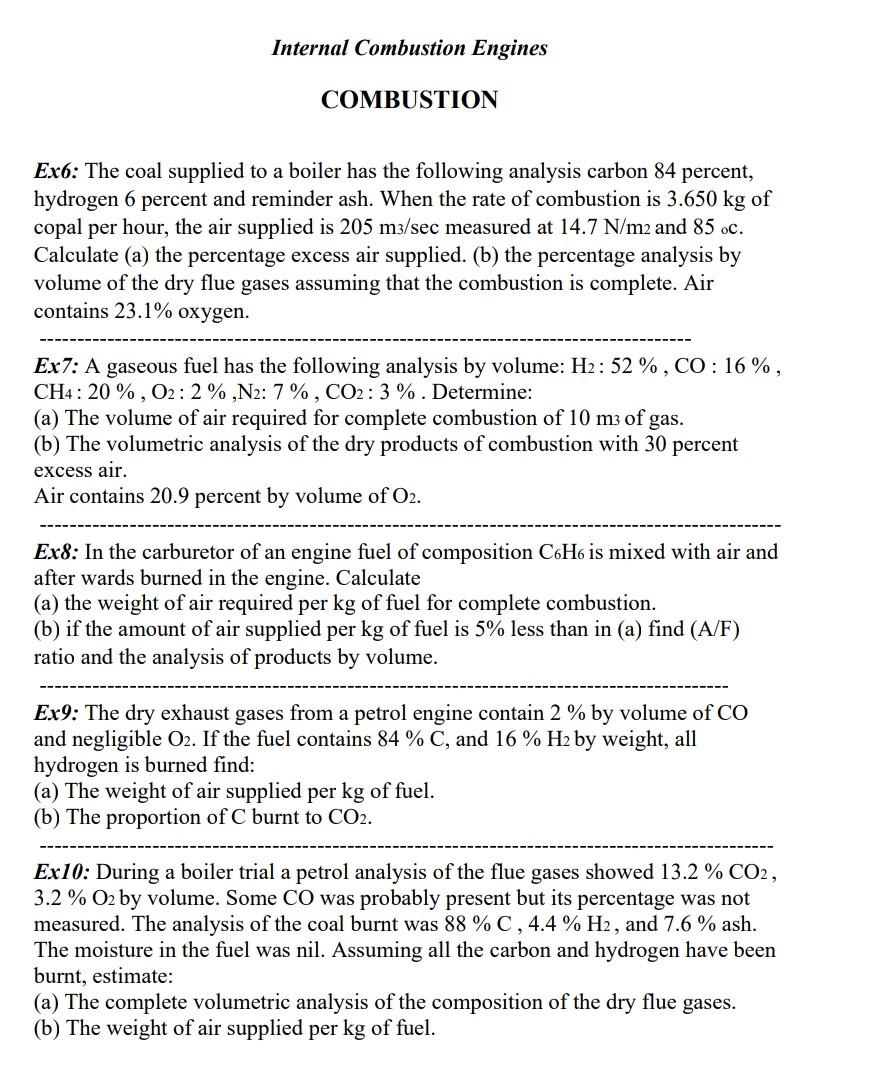
Internal Combustion Engines COMBUSTION Ex6: The coal supplied to a boiler has the following analysis carbon 84 percent, hydrogen 6 percent and reminder ash. When the rate of combustion is 3.650kg of copal per hour, the air supplied is 205m3/sec measured at 14.7N/m2 and 85 oc. Calculate (a) the percentage excess air supplied. (b) the percentage analysis by volume of the dry flue gases assuming that the combustion is complete. Air contains 23.1% oxygen. Ex7: A gaseous fuel has the following analysis by volume: H2:52%,CO:16%, CH4:20%,O2:2%,N2:7%,CO2:3%. Determine: (a) The volume of air required for complete combustion of 10m3 of gas. (b) The volumetric analysis of the dry products of combustion with 30 percent excess air. Air contains 20.9 percent by volume of O2. Ex8: In the carburetor of an engine fuel of composition C6H6 is mixed with air and after wards burned in the engine. Calculate (a) the weight of air required per kg of fuel for complete combustion. (b) if the amount of air supplied per kg of fuel is 5% less than in (a) find (A/F) ratio and the analysis of products by volume. Ex9: The dry exhaust gases from a petrol engine contain 2% by volume of CO and negligible O2. If the fuel contains 84%C, and 16%H2 by weight, all hydrogen is burned find: (a) The weight of air supplied per kg of fuel. (b) The proportion of C burnt to CO2. Ex10: During a boiler trial a petrol analysis of the flue gases showed 13.2%CO2, 3.2%O2 by volume. Some CO was probably present but its percentage was not measured. The analysis of the coal burnt was 88%C,4.4%H2, and 7.6% ash. The moisture in the fuel was nil. Assuming all the carbon and hydrogen have been burnt, estimate: (a) The complete volumetric analysis of the composition of the dry flue gases. (b) The weight of air supplied per kg of fuel
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


