Answered step by step
Verified Expert Solution
Question
1 Approved Answer
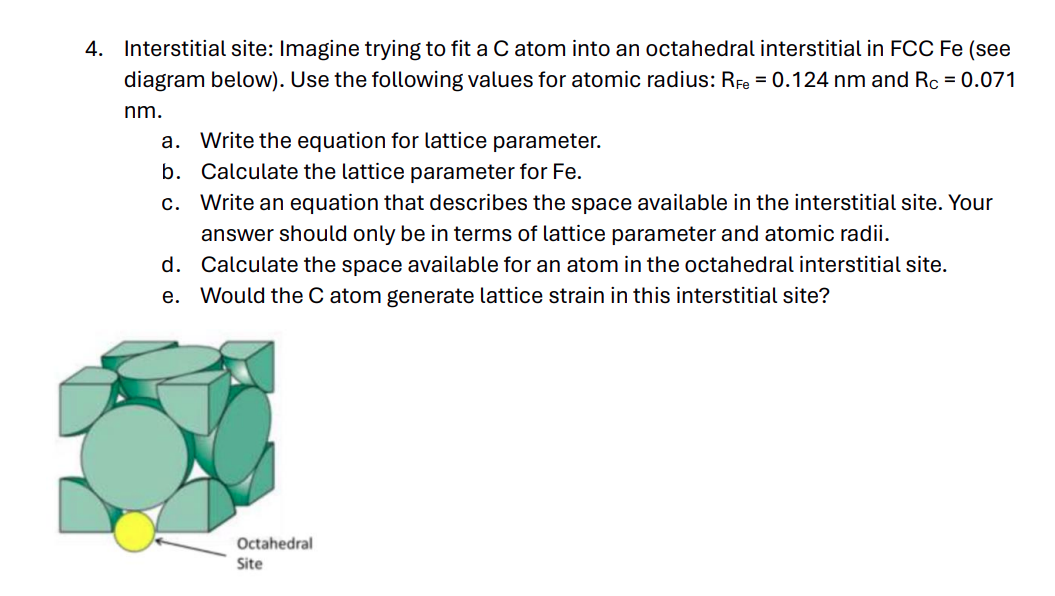
Interstitial site: Imagine trying to fit a C atom into an octahedral interstitial in FCC Fe ( see diagram below ) . Use the following
Interstitial site: Imagine trying to fit a C atom into an octahedral interstitial in FCC Fe see
diagram below Use the following values for atomic radius: RFe nm and RC
nm
a Write the equation for lattice parameter.
b Calculate the lattice parameter for Fe
c Write an equation that describes the space available in the interstitial site. Your
answer should only be in terms of lattice parameter and atomic radii.
d Calculate the space available for an atom in the octahedral interstitial site.
e Would the C atom generate lattice strain in this interstitial site?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


