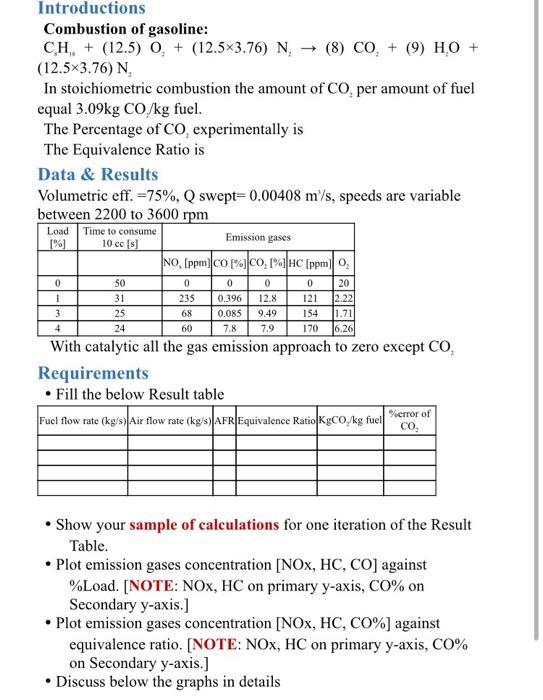
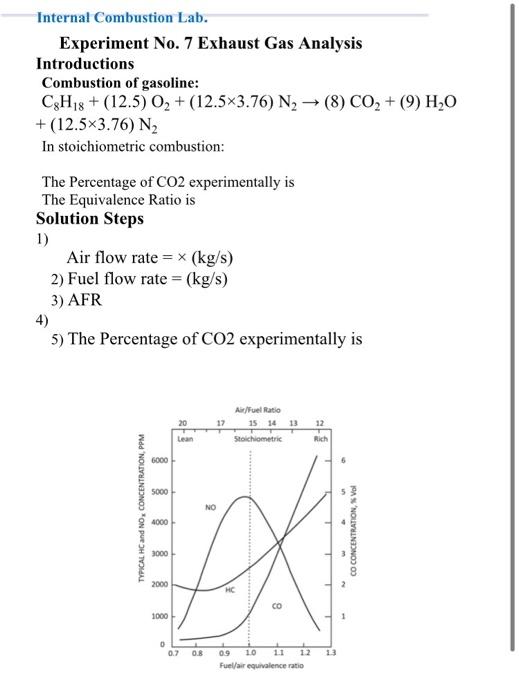
Introductions Combustion of gasoline: CH, + (12.5) O + (12.5x3.76) N (8) CO, + (9) HO + (12.5x3.76) N In stoichiometric combustion the amount of CO, per amount of fuel equal 3.09kg CO/kg fuel. The Percentage of Co, experimentally is The Equivalence Ratio is Data & Results Volumetric eff. =75%, swept=0.00408 m/s, speeds are variable between 2200 to 3600 rpm Load [%] Time to consume 10 cc (8) Emission gases NO, (ppm)CO [%]CO, HC (ppmo, 0 0 0 235 1 3 4 50 31 25 24 0.396 0.085 7.8 0 12.8 9.49 7.9 0 121 154 170 20 12.22 1.71 16.261 68 60 With catalytic all the gas emission approach to zero except CO, Requirements Fill the below Result table Fuel flow rate (kg/s Air flow rate (kg/s) |FR|Equivalence Ratio Kgco/kg fuel co %error of Show your sample of calculations for one iteration of the Result Table. Plot emission gases concentration (NOx, HC, CO) against %Load. [NOTE: NOX, HC on primary y-axis, Co% on Secondary y-axis.] Plot emission gases concentration [NOX, HC, CO%) against equivalence ratio. [NOTE: NOX, HC on primary y-axis, C0% on Secondary y-axis.] Discuss below the graphs in details Internal Combustion Lab. Experiment No. 7 Exhaust Gas Analysis Introductions Combustion of gasoline: C8H18 + (12.5) O2 + (12.5x3.76) N2 (8) CO2 + (9) H2O +(12.5x3.76) N2 In stoichiometric combustion: The Percentage of CO2 experimentally is The Equivalence Ratio is Solution Steps 1) Air flow rate = x (kg/s) 2) Fuel flow rate = (kg/s) 3) AFR 4) 5) The Percentage of CO2 experimentally is 17 Air/Fuel Ratio 15 14 13 Stoichiometric 12 Lean Rich 6000 5000 TYPICAL HC and NO, CONCENTRATION, PPM NO 4000 CO CONCENTRATION, Vol 3000 - 2000 - HC CO 1000 O 0.2 0.8 13 09 10 12 Fuel/air equivalence ratio Introductions Combustion of gasoline: CH, + (12.5) O + (12.5x3.76) N (8) CO, + (9) HO + (12.5x3.76) N In stoichiometric combustion the amount of CO, per amount of fuel equal 3.09kg CO/kg fuel. The Percentage of Co, experimentally is The Equivalence Ratio is Data & Results Volumetric eff. =75%, swept=0.00408 m/s, speeds are variable between 2200 to 3600 rpm Load [%] Time to consume 10 cc (8) Emission gases NO, (ppm)CO [%]CO, HC (ppmo, 0 0 0 235 1 3 4 50 31 25 24 0.396 0.085 7.8 0 12.8 9.49 7.9 0 121 154 170 20 12.22 1.71 16.261 68 60 With catalytic all the gas emission approach to zero except CO, Requirements Fill the below Result table Fuel flow rate (kg/s Air flow rate (kg/s) |FR|Equivalence Ratio Kgco/kg fuel co %error of Show your sample of calculations for one iteration of the Result Table. Plot emission gases concentration (NOx, HC, CO) against %Load. [NOTE: NOX, HC on primary y-axis, Co% on Secondary y-axis.] Plot emission gases concentration [NOX, HC, CO%) against equivalence ratio. [NOTE: NOX, HC on primary y-axis, C0% on Secondary y-axis.] Discuss below the graphs in details Internal Combustion Lab. Experiment No. 7 Exhaust Gas Analysis Introductions Combustion of gasoline: C8H18 + (12.5) O2 + (12.5x3.76) N2 (8) CO2 + (9) H2O +(12.5x3.76) N2 In stoichiometric combustion: The Percentage of CO2 experimentally is The Equivalence Ratio is Solution Steps 1) Air flow rate = x (kg/s) 2) Fuel flow rate = (kg/s) 3) AFR 4) 5) The Percentage of CO2 experimentally is 17 Air/Fuel Ratio 15 14 13 Stoichiometric 12 Lean Rich 6000 5000 TYPICAL HC and NO, CONCENTRATION, PPM NO 4000 CO CONCENTRATION, Vol 3000 - 2000 - HC CO 1000 O 0.2 0.8 13 09 10 12 Fuel/air equivalence ratio