Question
Irreversible reactions 1 and 2 occur in an adiabatic batch reactor. The solution can be treated as ideal with a constant heat capacity of 1.23
Irreversible reactions 1 and 2 occur in an adiabatic batch reactor. The solution can be treated as ideal with a constant heat capacity of 1.23 cal/(g K) and a constant density of 1.02g/cm3.
A + B C + D (1)
C + B E + F (2)
The reactor is charged with 1900 L of a solution at 180C containing A and B with concentrations of CAo=2.9 mol/L and CBo=3.2 mol/L. A small amount of catalyst is added to the reactor. determine the reaction time for the concentration of D to reach a concentration of 1.0 mol/L. determine the concentrations of the other species and the temperature of the reactor.
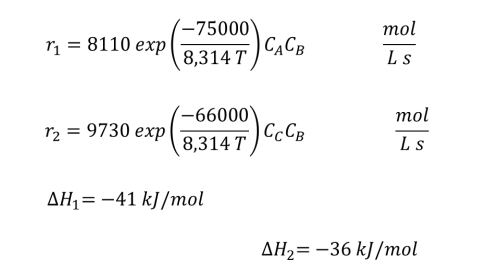
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


