Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Isomers and Resonance Structures 1.46 Creatine is a dietary supplement used by some athletes to boost their athletic performance. (a) Draw in all lone pairs
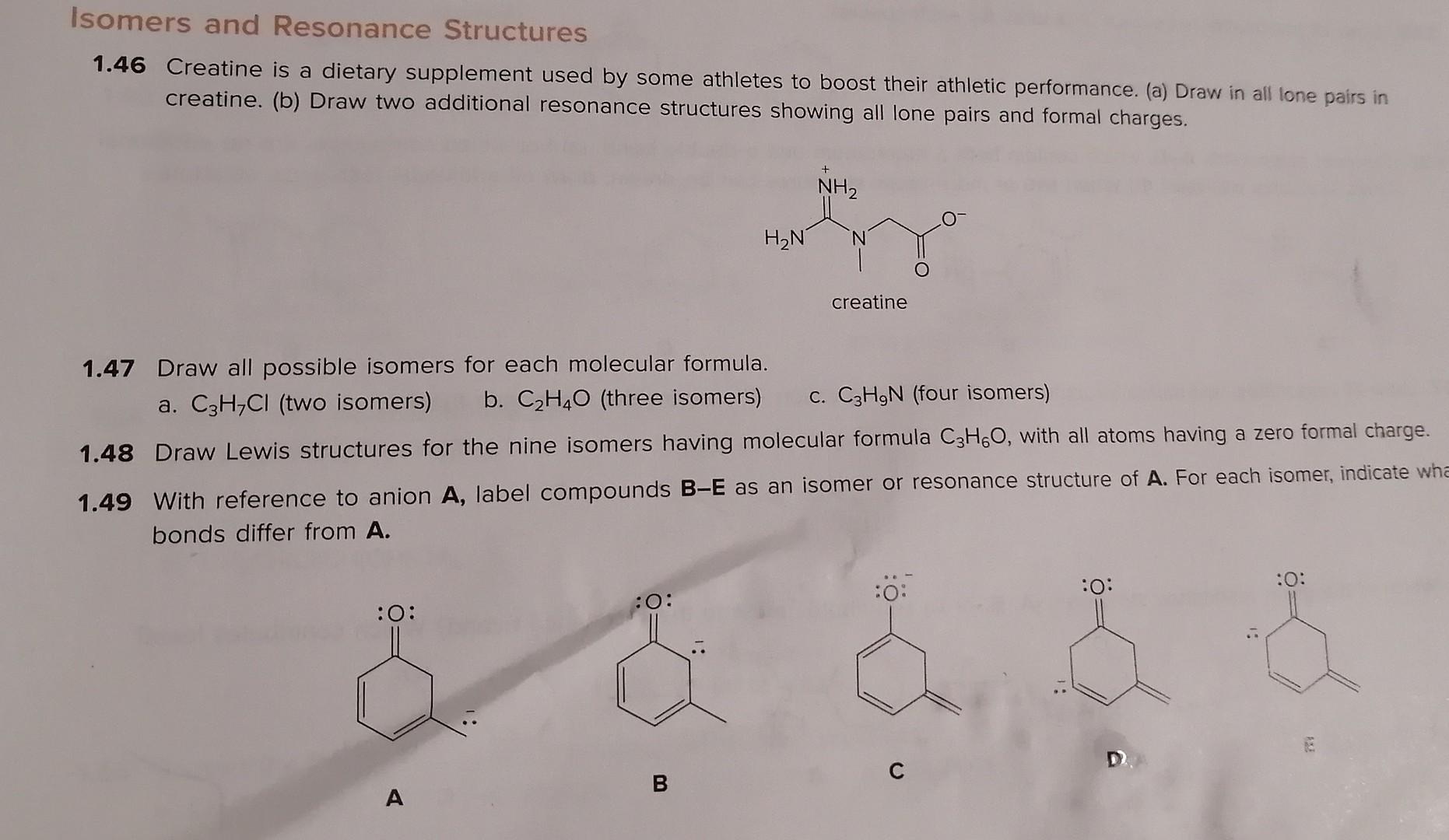
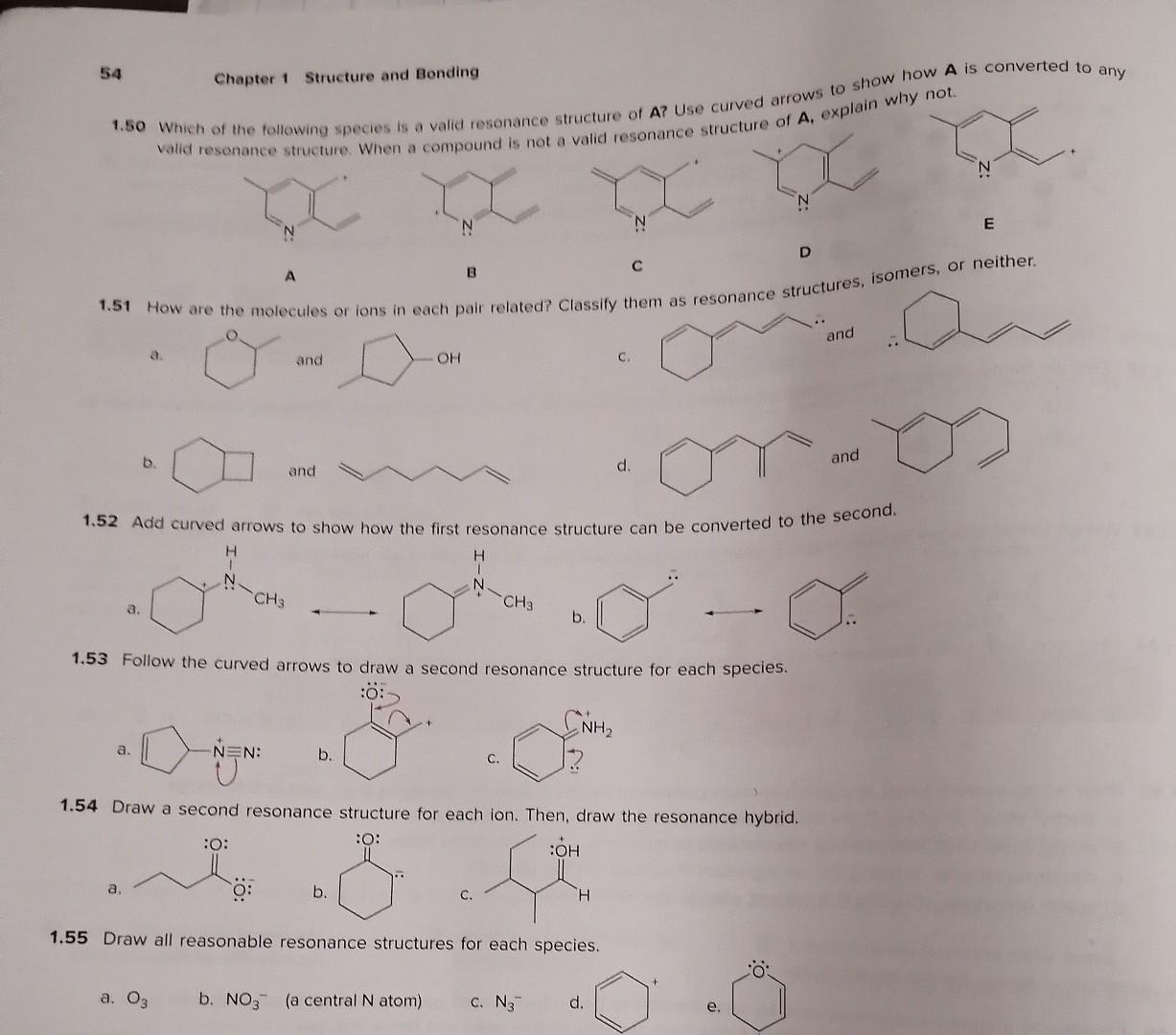
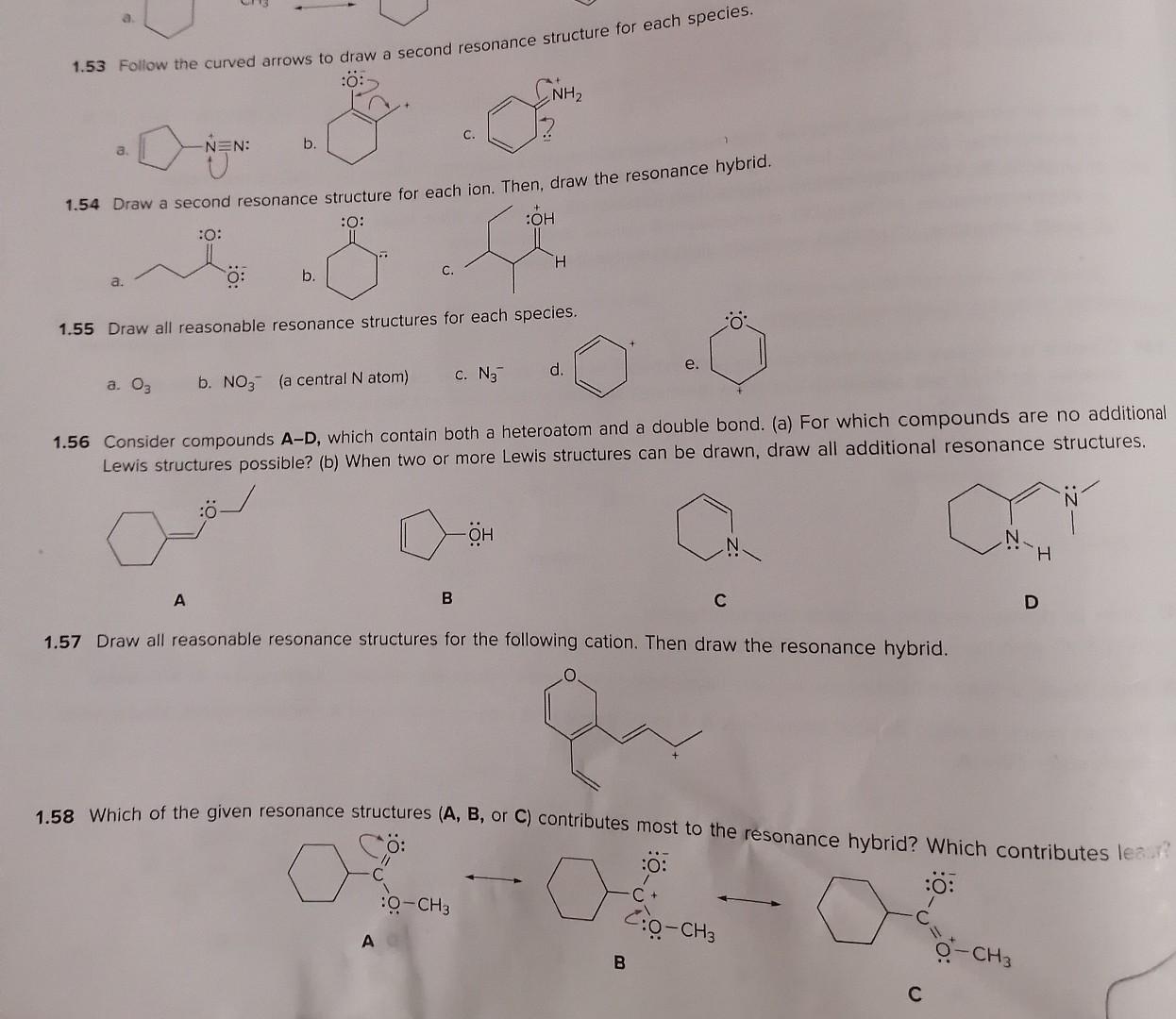
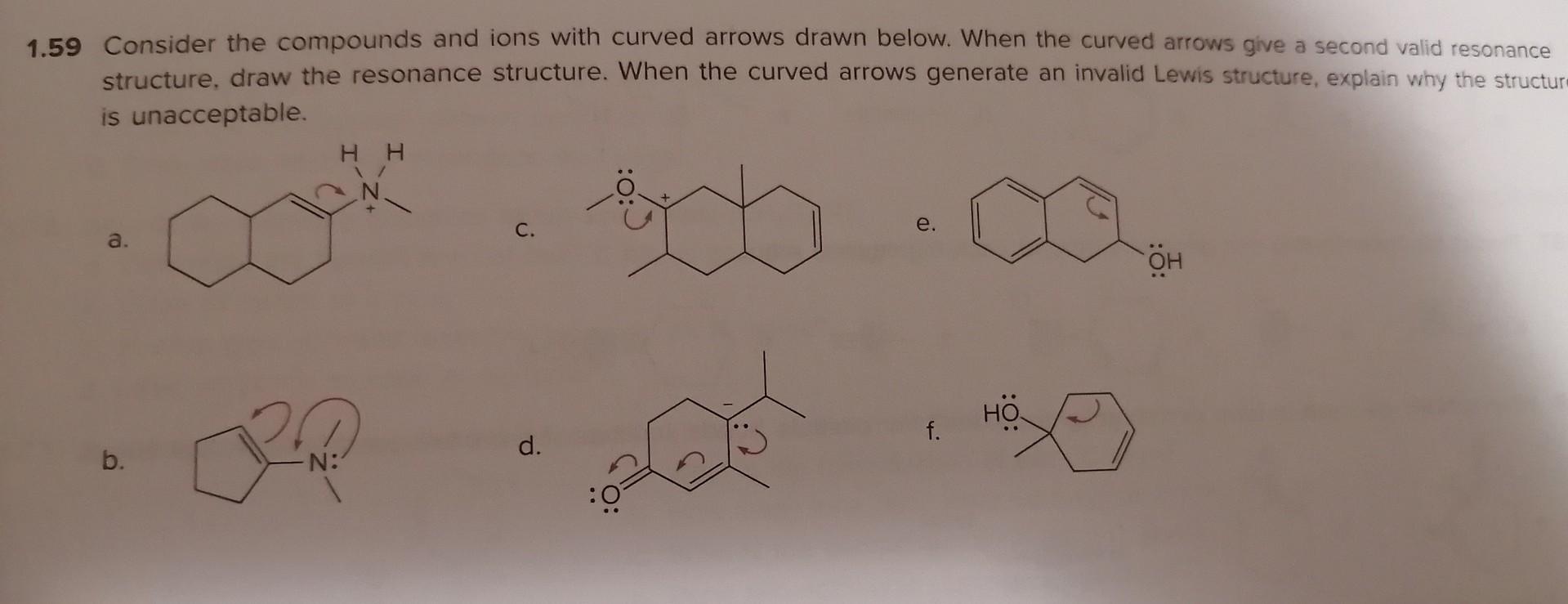
Isomers and Resonance Structures 1.46 Creatine is a dietary supplement used by some athletes to boost their athletic performance. (a) Draw in all lone pairs in creatine. (b) Draw two additional resonance structures showing all lone pairs and formal charges. creatine 1.47 Draw all possible isomers for each molecular formula. a. C3H7Cl (two isomers) b. C2H4O (three isomers) c. C3H9N (four isomers) 1.48 Draw Lewis structures for the nine isomers having molecular formula C3H6O, with all atoms having a zero formal charge. 1.49 With reference to anion A, label compounds BE as an isomer or resonance structure of A. For each isomer, indicate wh bonds differ from A. A B C D. 54 Chapter 1 Structure and Bonding 1.50 Which of the following spectes is a valid resonance structure of A? Use curved arrows to show how valid resonance structure. When a compound is not a valid resonance structure of A, explain why not. A B C D 1.51 How are the molecules or lons in each pair related? Classify them as resonance structures, isomers, or neither. a. and c. and b. and d. and 1.52 Add curved arrows to show how the first resonance structure can be converted to the second. a. b. c. a. b. 1.54 Draw a second resonance structure for each ion. Then, draw the resonance hybrid. a. b. c. 1.55 Draw all reasonable resonance structures for each species. a. O3 b. NO3(a central N atom) C. N3 d. e. a. 1.53 Follow the curved arrows to draw a second resonance structure for each species. a. b. c. 1.54 Draw a second resonance structure for each ion. Then, draw the resonance hybrid. a. b. 1.55 Draw all reasonable resonance structures for each species. a. O3 b. NO3(a central N atom) c. N3 d. e. 1.56 Consider compounds A-D, which contain both a heteroatom and a double bond. (a) For which compounds are no addional Lewis structures possible? (b) When two or more Lewis structures can be drawn, draw all additional resonance structures. A B C D 1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid. 1.58 Which of the given resonance structures (A,B, or C) contributes most to the resonance hybrid? Which contributes les. 1.59 Consider the compounds and ions with curved arrows drawn below. When the curved arrows give a second valid resonance structure, draw the resonance structure. When the curved arrows generate an invalid Lewis structure, explain why the structur is unacceptable. a. c. e. b. d. f
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


