Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Isotope vs Isotopologue abundance calculate the mole fraction abundance (in percent) of the most abundant isotopologue of CO2 (~mass 44) Show all work please and
Isotope vs Isotopologue abundance
calculate the mole fraction abundance (in percent) of the most abundant isotopologue of CO2 (~mass 44)
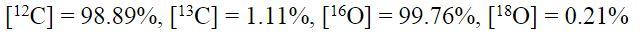
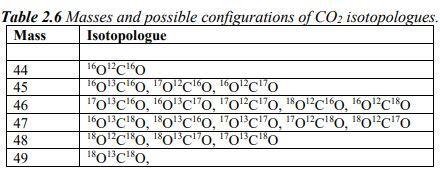
Show all work please and thank you!
[12C] = 98.89%, [13C] = 1.11%,[160] = 99.76%, [180] = 0.21% = = Table 2.6 Masses and possible configurations of CO2 isotopologues. Mass Isotopologue 17013 16 18 160 44 45 46 47 48 49 160'c160 160C 60, 10C160, 160CHO To'clo, o'clo, Toclo, foclo, o'clo 160'c180, 180'C', 'O'C''O, "O'Clo, o'c'o 180'CO, "O'C'O, "O'CO , 17 18 180 1813 18 [12C] = 98.89%, [13C] = 1.11%,[160] = 99.76%, [180] = 0.21% = = Table 2.6 Masses and possible configurations of CO2 isotopologues. Mass Isotopologue 17013 16 18 160 44 45 46 47 48 49 160'c160 160C 60, 10C160, 160CHO To'clo, o'clo, Toclo, foclo, o'clo 160'c180, 180'C', 'O'C''O, "O'Clo, o'c'o 180'CO, "O'C'O, "O'CO , 17 18 180 1813 18Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


