Answered step by step
Verified Expert Solution
Question
1 Approved Answer
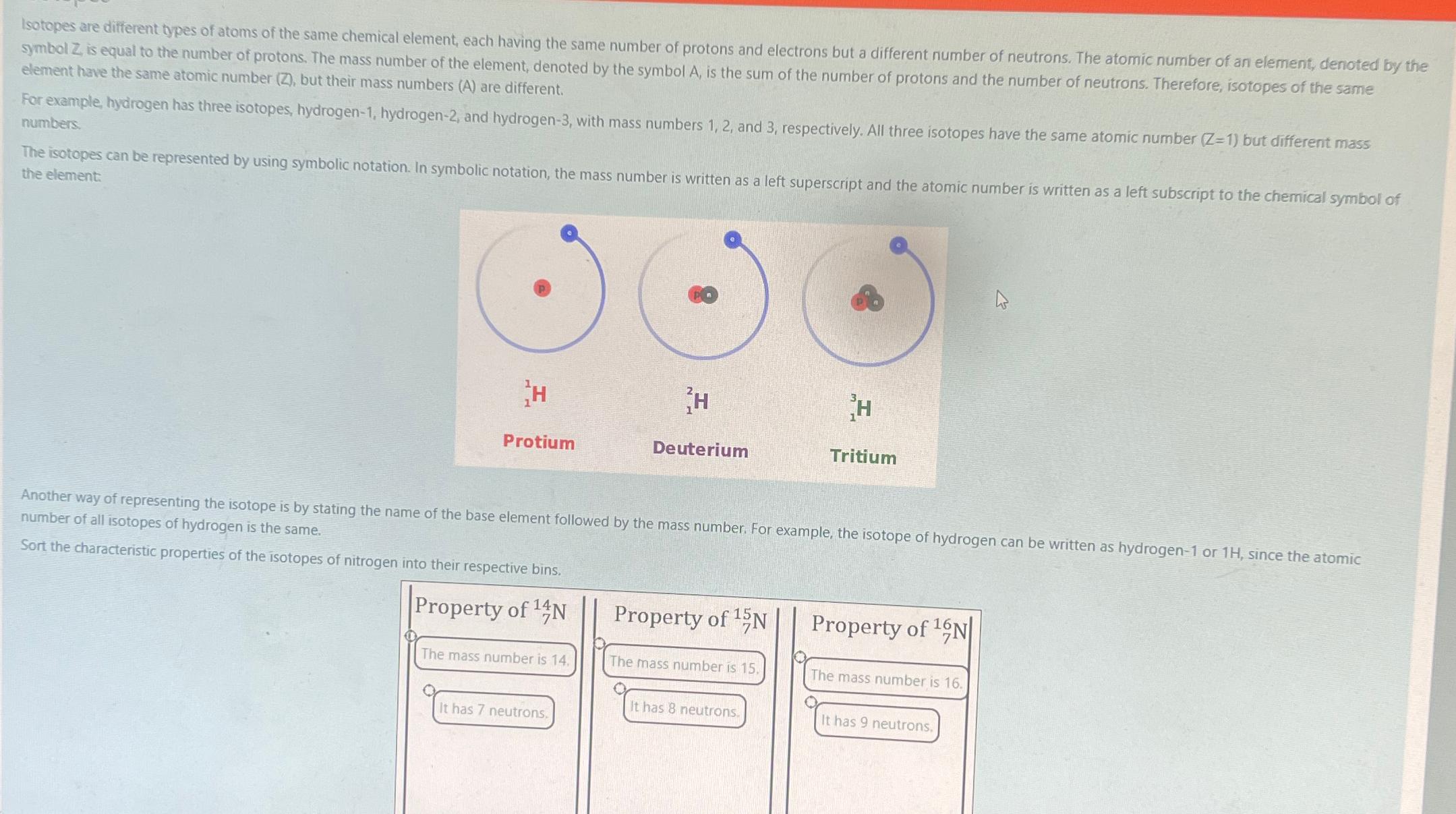
Isotopes are different types of atoms of the same chemical element, each having the same number of protons and electrons but a different number of
Isotopes are different types of atoms of the same chemical element, each having the same number of protons and electrons but a different number of neutrons. The atomic number of an element, denoted by the symbol is equal to the number of protons. The mass number of the element, denoted by the symbol is the sum of the number of protons and the number of neutrons. Therefore, isotopes of the same element have the same atomic number but their mass numbers are different.
For example, hydrogen has three isotopes, hydrogen hydrogen and hydrogen with mass numbers and respectively. All three isotopes have the same atomic number but different mass numbers.
The isotopes can be represented by using symbolic notation. In symbolic notation, the mass number is written as a left superscript and the atomic number is written as a left subscript to the chemical symbol of the element:
Another way of representing the isotope is by stating the name of the base element followed by the mass number. For example, the isotope of hydrogen can be written as hydrogen or since the atomic number of all isotopes of hydrogen is the same.
Sort the characteristic properties of the isotopes of nitrogen into their respective bins. The answers I have entered are partially right!! out of point
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


