Answered step by step
Verified Expert Solution
Question
1 Approved Answer
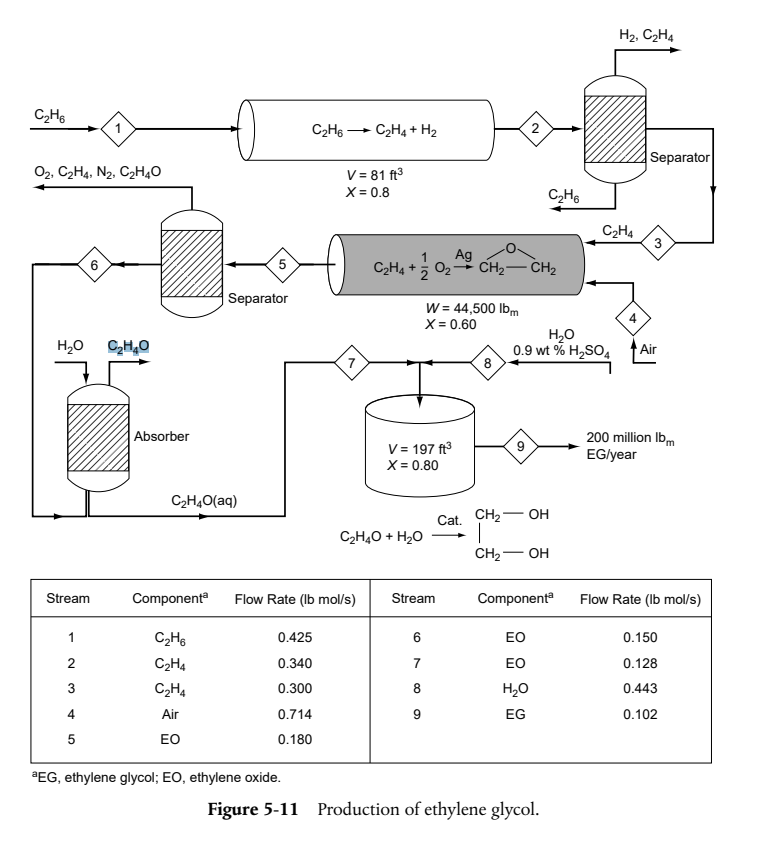
It is desired to produce 200 million pounds per year of EG. The reactor is to be operated isothermally. A 1 lbmol/frs aqueous solution of
It is desired to produce 200 million pounds per year of EG. The reactor is to be operated isothermally. A 1 lbmol/frs aqueous solution of ethylene oxide (EO) is fed to the reactor together with an equal volumetric solution of water containing 0.9 wt% of the catalyst H2SO4
DO MATERIAL BALANCE ( lb-mol/s) USING EXTINCT OF REACTION STARTING FROM STREAM 9
please dont copy
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


