Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ive tried to get help on this, but everupne ends up giving me the same answer i gave on my homework. the answer i gave
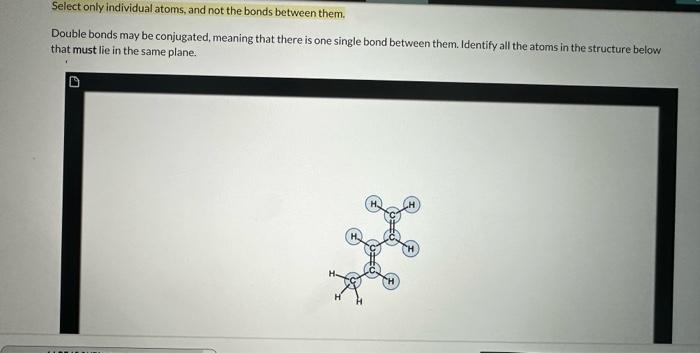

ive tried to get help on this, but everupne ends up giving me the same answer i gave on my homework. the answer i gave in the girst picture is wrong. i tried all the atoms except for the three hydrogens connected to the c at the bottom, but it isn't correct. in the second picture is the hint that im given when i got the answer i gave and it turned out to be wrong. im just confused on what would happen to the molecule planerly if the single bond between the two double bonds can rotate freely.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


