Answered step by step
Verified Expert Solution
Question
1 Approved Answer
J. Franckaerts and G. F. Froment [Chem. Eng. Sci., 19 (1964) 807] investigated the reaction listed in Exercise 3 over the same temperature range using
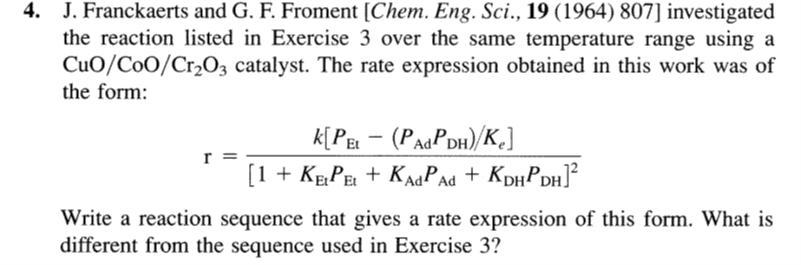 J. Franckaerts and G. F. Froment [Chem. Eng. Sci., 19 (1964) 807] investigated the reaction listed in Exercise 3 over the same temperature range using a CuO/CoO/Cr2O3 catalyst. The rate expression obtained in this work was of the form: r=[1+KEtPEt+KAdPAd+KDHPDH]2k[PEt(PAdPDH)/Ke] Write a reaction sequence that gives a rate expression of this form. What is different from the sequence used in Exercise 3
J. Franckaerts and G. F. Froment [Chem. Eng. Sci., 19 (1964) 807] investigated the reaction listed in Exercise 3 over the same temperature range using a CuO/CoO/Cr2O3 catalyst. The rate expression obtained in this work was of the form: r=[1+KEtPEt+KAdPAd+KDHPDH]2k[PEt(PAdPDH)/Ke] Write a reaction sequence that gives a rate expression of this form. What is different from the sequence used in Exercise 3 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


