Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Java Programming. Just code needed, not answers to question. Please give me the code for this program all the way up to the step where
Java Programming. Just code needed, not answers to question. Please give me the code for this program all the way up to the step where it asks "repeat this for l = 1, 2, 3".
Thank you!
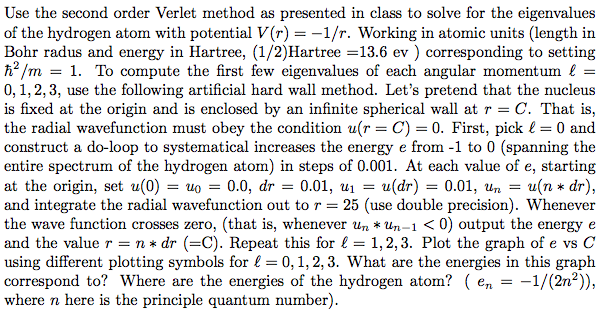
Use the second order Verlet method as presented in class to solve for the eigenvalues of the hydrogen atom with potential V(r) 1/r. Working in atomic units (length in Bohr radus and energy in Hartree, (1/2)Hartree 13.6 ev corresponding to setting h /m 1. To compute the first few eigenvalues of each angular momentum l 0, 1, 2, 3, use the following artificial hard wall method. Let's pretend that the nucleus is fixed at the origin and is enclosed by an infinite spherical wall at r C. That is, the radial wavefunction must obey the condition w(r C) 30. First, pick l 0 and construct a do-loop to systematical increases the energy e from -1 to 0 (spanning the entire spectrum of the hydrogen atom) in steps of 0.001. At each value of e, starting at the origin, set u(0) uo 0.0, dr 0.01, u u (dr) 0.01, un u(n dr), and integrate the radial wavefunction out to r 25 (use double precision). Whenever the wave function crosses zero, (that is, whenever un um-1 0) output the energy e and the value r n dr C). Repeat this for l 1, 2, 3. Plot the graph of e vs C using different plotting symbols for l 0, 1, 2, 3. What are the energies in this graph correspond to? Where are the energies of the hydrogen atom? en 1/(2n2)) where n here is the principle quantum number) Use the second order Verlet method as presented in class to solve for the eigenvalues of the hydrogen atom with potential V(r) 1/r. Working in atomic units (length in Bohr radus and energy in Hartree, (1/2)Hartree 13.6 ev corresponding to setting h /m 1. To compute the first few eigenvalues of each angular momentum l 0, 1, 2, 3, use the following artificial hard wall method. Let's pretend that the nucleus is fixed at the origin and is enclosed by an infinite spherical wall at r C. That is, the radial wavefunction must obey the condition w(r C) 30. First, pick l 0 and construct a do-loop to systematical increases the energy e from -1 to 0 (spanning the entire spectrum of the hydrogen atom) in steps of 0.001. At each value of e, starting at the origin, set u(0) uo 0.0, dr 0.01, u u (dr) 0.01, un u(n dr), and integrate the radial wavefunction out to r 25 (use double precision). Whenever the wave function crosses zero, (that is, whenever un um-1 0) output the energy e and the value r n dr C). Repeat this for l 1, 2, 3. Plot the graph of e vs C using different plotting symbols for l 0, 1, 2, 3. What are the energies in this graph correspond to? Where are the energies of the hydrogen atom? en 1/(2n2)) where n here is the principle quantum number)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


