Answered step by step
Verified Expert Solution
Question
1 Approved Answer
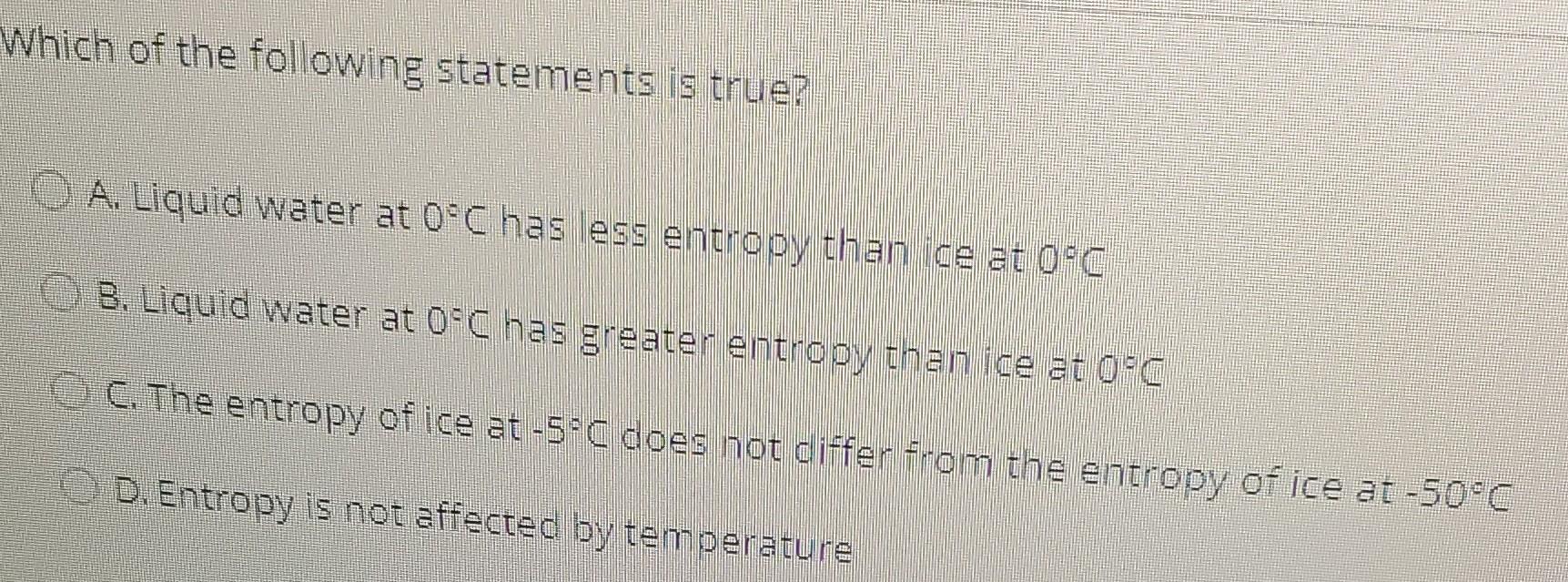
just enclose the answer por each question thank you Which of the following statements is true? A. Liquid water at 0C has less entropy than


just enclose the answer por each question thank you
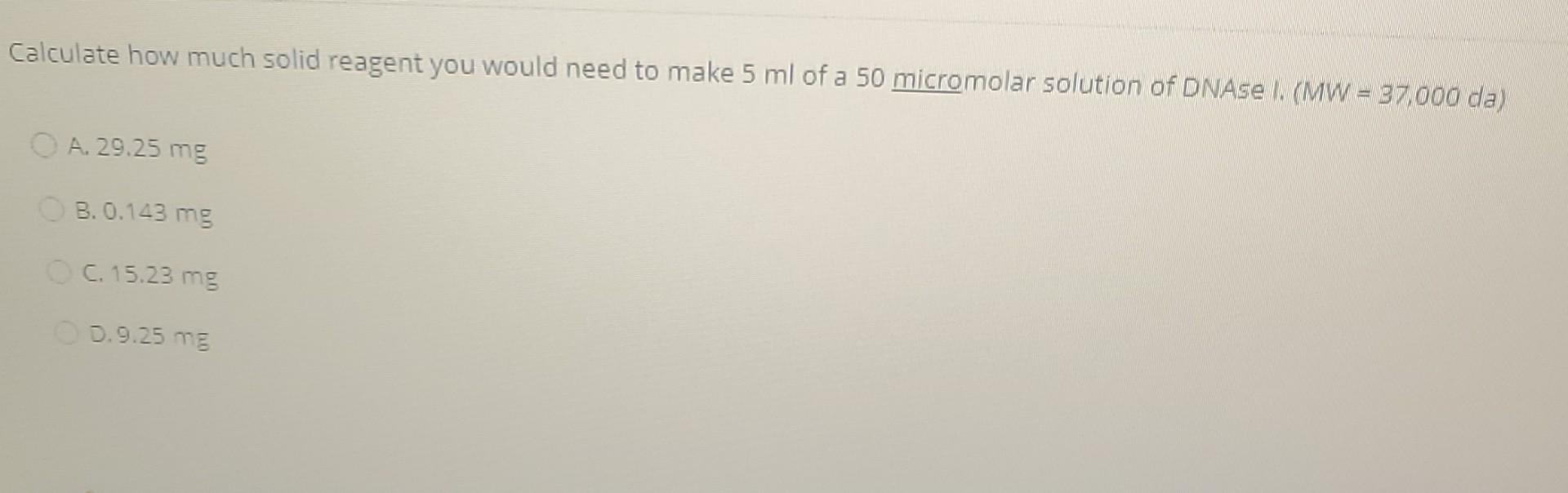
Which of the following statements is true? A. Liquid water at 0C has less entropy than ice at 0C 3. Liquid water at 0C has greater entropy than ice at 0C C. The entropy of ice at -5C does not differ from the entropy of ice at -50C D. Entropy is not affected by temoerature Calculate how much solid reagent you would need to make 5 ml of a 50 micromolar solution of DNAse (MW = 37.000 da) O A. 29.25 mg B. 0.143 mg C. 15.23 mg 0.9.25 mg When the reaction A+B --> 2C is at equilibrium, the concentrations of reactants are as follows: (A) = 8 mM [B] = 9 mm and [C] = 12 mm. What is the standard free energy change for the reaction? A. 4439) . -4439) C.-1717) D. 1717) Which is not one of the four thermodynamic state functions? A. Energy, U B. Entha py. H C. Entropy D. Dissociation constant. KoStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


