Answered step by step
Verified Expert Solution
Question
1 Approved Answer
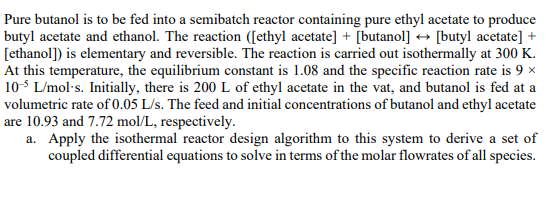
Just part C please Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol. The

Just part C please
Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol. The reaction ([ethyl acetate] +[ butanol ] [butyl acetate] + [ethanol]) is elementary and reversible. The reaction is carried out isothermally at 300K. At this temperature, the equilibrium constant is 1.08 and the specific reaction rate is 9 105L/mols. Initially, there is 200L of ethyl acetate in the vat, and butanol is fed at a volumetric rate of 0.05L/s. The feed and initial concentrations of butanol and ethyl acetate are 10.93 and 7.72mol/L, respectively. a. Apply the isothermal reactor design algorithm to this system to derive a set of coupled differential equations to solve in terms of the molar flowrates of all species. c. Repeat part (a) to include ethanol evaporation (reactive distillation) as soon as it forms. Do you think the inclusion of reactive distillation will improve or hinder the overall reaction? Conceptually discuss and justify your responseStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


