Answered step by step
Verified Expert Solution
Question
1 Approved Answer
kindly solve in 30 minutes, i will give you two likes D Question 7 3 pts Hydrogen Emission Spectrum 1 1 700nm 400nm The figure
kindly solve in 30 minutes, i will give you two likes 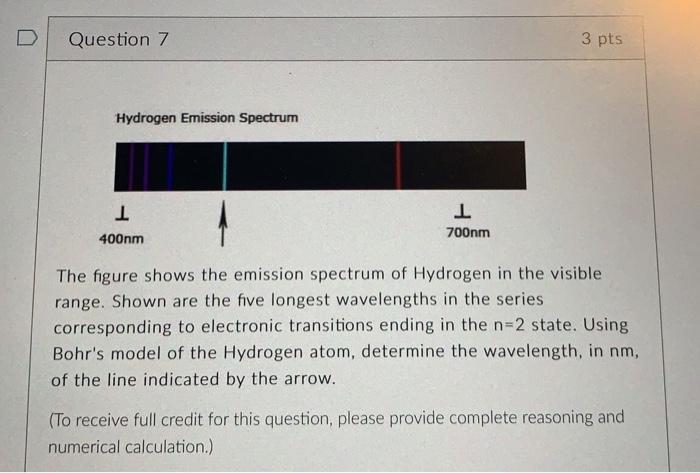
D Question 7 3 pts Hydrogen Emission Spectrum 1 1 700nm 400nm The figure shows the emission spectrum of Hydrogen in the visible range. Shown are the five longest wavelengths in the series corresponding to electronic transitions ending in the n=2 state. Using Bohr's model of the Hydrogen atom, determine the wavelength, in nm, of the line indicated by the arrow. (To receive full credit for this question, please provide complete reasoning and numerical calculation.) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


