Question
n 13. According to the following activity series: Mg-Zn-Fe-Pb-H-Cu (- decrease reactivity) Which of the following equations show no reactions? (1) Fe(s) + PbSO4 (aq)
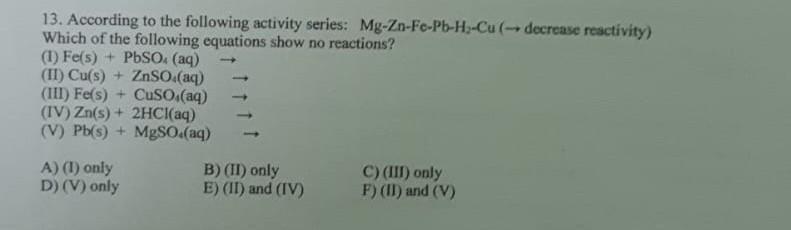 n
n
13. According to the following activity series: Mg-Zn-Fe-Pb-H-Cu (- decrease reactivity) Which of the following equations show no reactions? (1) Fe(s) + PbSO4 (aq) (II) Cu(s) + ZnSO.(aq) (III) Fe(s) + CuSO.(aq) (IV) Zn(s) + 2HCl(aq) (V) Pb(s) + MgSO.(aq) A) (1) only D) (V) only B) (II) only E) (II) and (IV) C) (III) only F) (II) and (V)
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
A displacement reaction occurs when a reactive ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Understandable Statistics Concepts And Methods
Authors: Charles Henry Brase, Corrinne Pellillo Brase
12th Edition
1337119911, 978-1337517508, 133751750X, 978-1337119917
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



