Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) Calculate the Gibbs free energy (AG) of above reaction (last two digit of your student number 10) C? b) Calculate the equilibrium constant
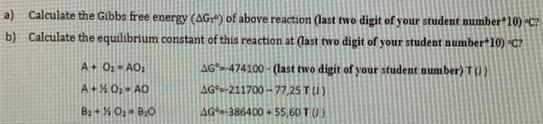
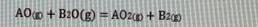
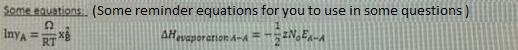
a) Calculate the Gibbs free energy (AG) of above reaction (last two digit of your student number 10) C? b) Calculate the equilibrium constant of this reaction at (last two digit of your student number 10) C? A+ O AO AG-474100 - (last two digit of your student nunmber) T(1) A+X 0: AO AG-211700 -77,25 TU) Ba + % O: BO AG-386400 + 55,60 T () AOo + B20(g) = AOzp + B20 Some equations: (Some reminder equations for you to use in some questions) InyA = RT AHvaporarion A-A
Step by Step Solution
★★★★★
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
a 10...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635f2ca77764a_231268.pdf
180 KBs PDF File
635f2ca77764a_231268.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


