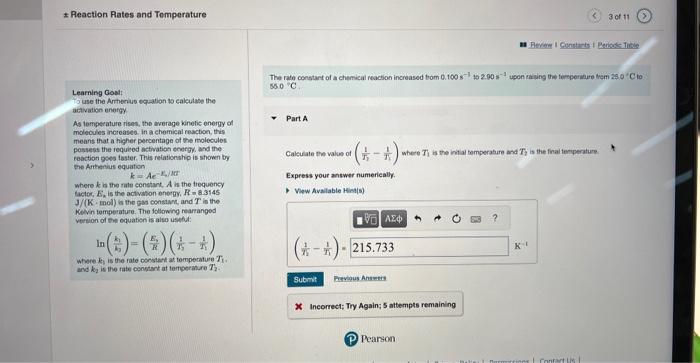
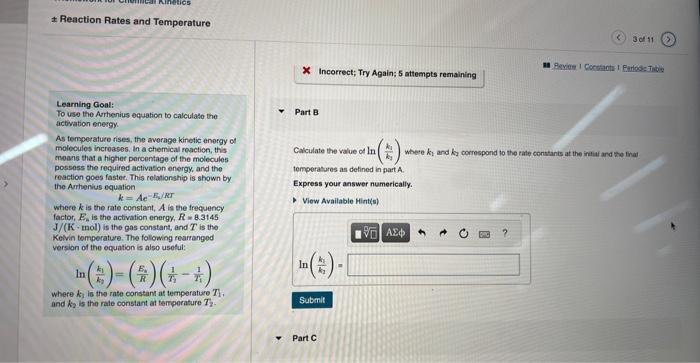
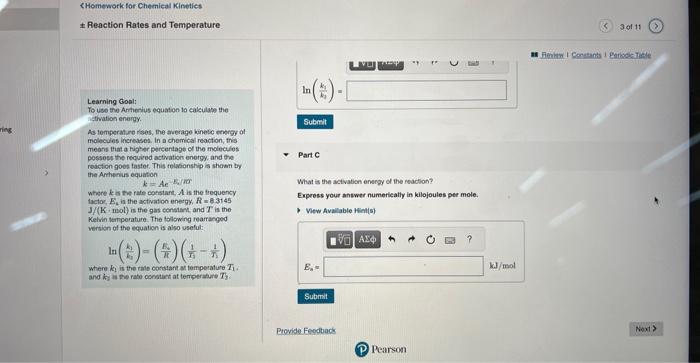
Learning Gostt The rate constart of a chemical reacion increased trom 0.100s1 is 2.90s1 upon ending the fereperiare tremi 24.01C to To use the Armentus tquation to calculase the artivatien energy As temperature rises, the ewerage kinetic energy of Part A molecules increasec. in a chemical reaction, this means that a higher percentage of the molecules possess the requeded activation energy. and the: reaction goes taster. This reiationship is shown by the Antranius equation where k is the rate constant, A is the trequency tacter, En is the actlyabon eneetr. R=8.3145 J/(K. mol ) a the gas constan, and T is the Kelin temperature. The following rearanged vereion of eve equation is alsa usetid: In(k3k1)=(kE3)(T31T11) where K3 is the rate corsthet at tomperature T1. and k2 is the rate conptant at terteerature T2. X. Incorrect; Try Again; 5 attempts remaining Reaction Rates and Temperature X incorrect; Try Again; 5 attompts remaining Learning Goal: To uso the Ampenius equation to calculaso the Part B activation energy. As temperature rises, the avorage kinotic enorgy of molocules incroases, in a chemical reaction, this moans that a higher parcentage of the molecules possoss the required activation energy, and the resetion goes fastec. This relationship is shown by the Arthentus equation k=AeE2/RT where k is the rate constant, A is the frequency factor, En is the activation energy, R=8.3145 J/(K. mol) is the gas constant, and T is the Kevin tomperatue. The following rearrangod version of the equation is also usoful: ln(kik1)=(REi)(T11T11) Cavculate the value of ln(k1k1) whee k1 and k2 corrospond to the rale constants at the intut and to inat temporausus as defined in part A. Express your answer numerically. where k2 is the rate constant at temperature T1. and k2 is the rate constant at terperature T2. Reaction Rates and Temperature Learning Gool: ln(k1k1)= To use the Antherius equaton to calculape the Atthration enargy. As tomperature rises, the average kinete energy of molecules increases. In a chemical reaction, this meane that a higher persentage of the molecules possees the required actwation energy, and the Part 6 reaction goos taster. This rolasonship is shawit by the Anhenus equation k=Aeir What is the activation energy of the reaction? where k is the fate cotstant, A is the trequency Iactor, E4 in the activation energy, R=8.3145 Jf(K,mol) is the gas censtart, and T is the Kelvin temporature. The following rearranged versien of the equatien is also useful: ln(k1k1)=(r1R4)(r11r11) Expeess your answer numertealiy in kilojoules pet mole. where k1 is the tale condtant at temperature T1. and k in in tie rate conaticst at terreerahare T2