Answered step by step
Verified Expert Solution
Question
1 Approved Answer
looking for mololity of unknown, moles of unonown, molar mass unknow the other pictures are information in celcius TRIAL 1 Assume a van't Hoff factor,
looking for mololity of unknown, moles of unonown, molar mass unknow
the other pictures are information 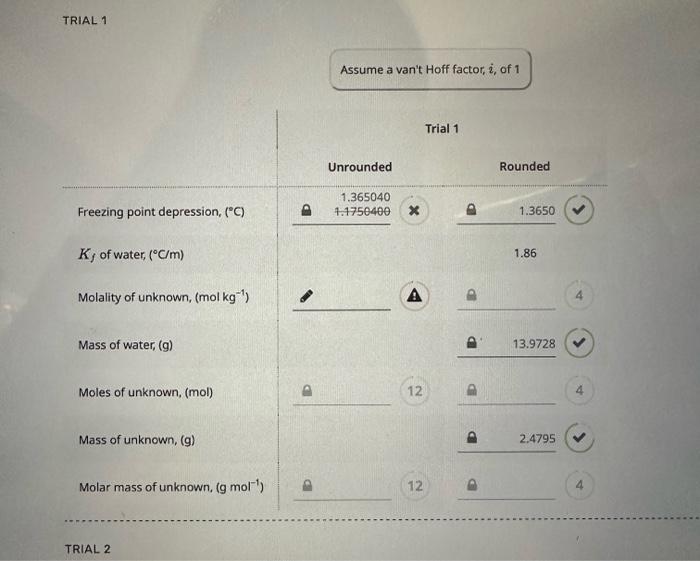
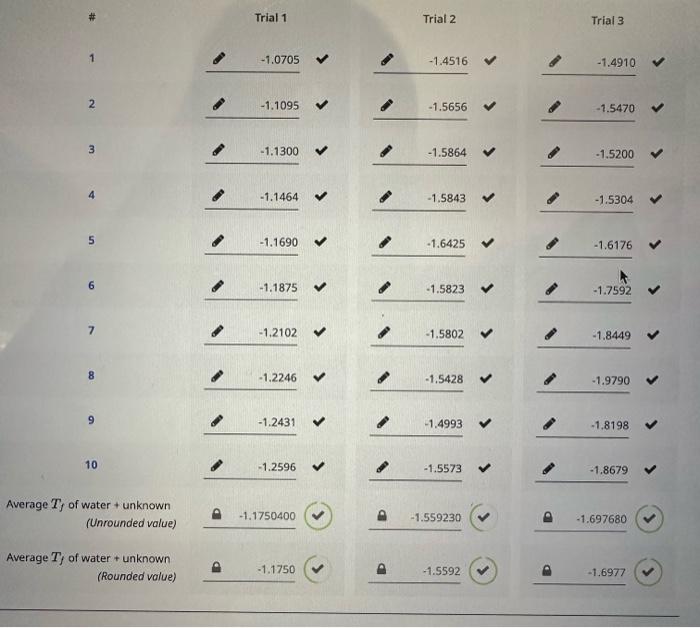
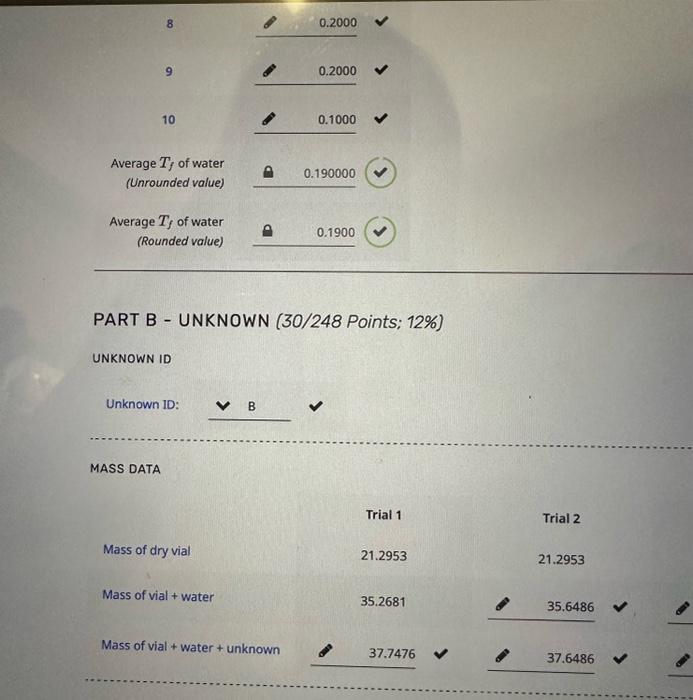



in celcius
TRIAL 1 Assume a van't Hoff factor, i, of 1 Trial 1 Freezing point depression, (C) Kf of water, (C/m) Rounded Unrounded 1.3650401.1750400 1.3650 Molality of unknown, (molkg1) Mass of water, (g) Moles of unknown, (mol) Mass of unknown, (g) Molar mass of unknown, (gmol1) 4 12 A 4 TRIAL 2 \# Trial 1 Trial 2 Trial 3 1 2 1.1095+1.56561.5470 3 4 5 6 7 1.5802 1.8449 8 9 10 Average Tf of water + unknown water + unknown (Unrounded value) Average Tf of water + unknown 8 0.2000 9 0.2000 10 0.1000 Average Tf of water (Unrounded value) 0.190000 Average Tf of water (Rounded value) 0.1900 PART B - UNKNOWN (30/248 Points; 12\%) UNKNOWN ID Unknown 1D: MASS DATA Trial 1 Trial 2 Mass of dry vial 21.295321.2953 Mass of vial + water 35.2681 Mass of vial + water + unknown 37.747637.6486 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


