Question
When 4.90 g of a nonelectrolyte solute is dissolved in water to make 555 ml of solution at 21 C, the solution exerts an
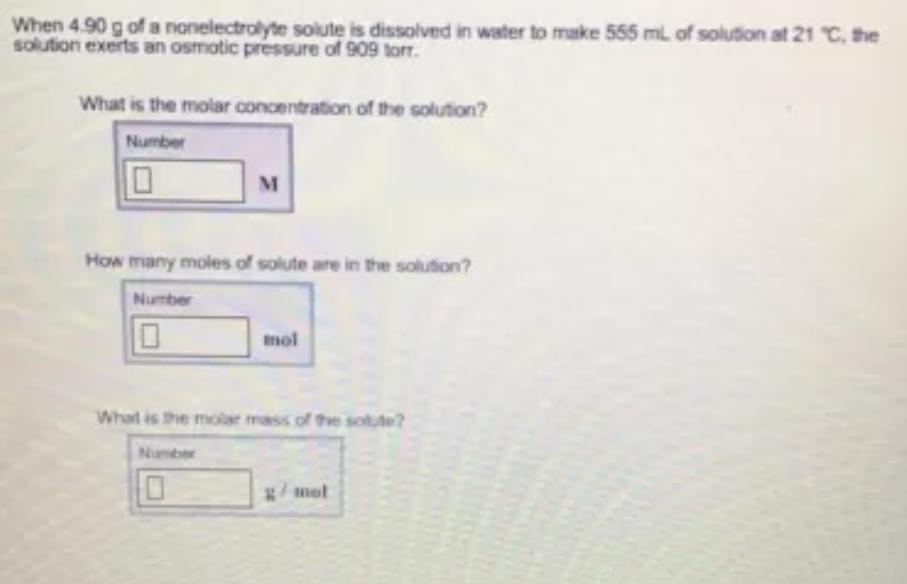
When 4.90 g of a nonelectrolyte solute is dissolved in water to make 555 ml of solution at 21 C, the solution exerts an osmotic pressure of 909 torr. What is the molar concentration of the solution? Number M How many moles of solute are in the solution? Number mol Whal is the molar mass of thhe solute? Number g/mot
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
Given m 490que 120 atm OS motic pessre q09 toY T 21C 294 K O Si...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Mathematical Statistics And Its Applications
Authors: Richard J. Larsen, Morris L. Marx
5th Edition
321693949, 978-0321694027, 321694023, 978-0321693945
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



