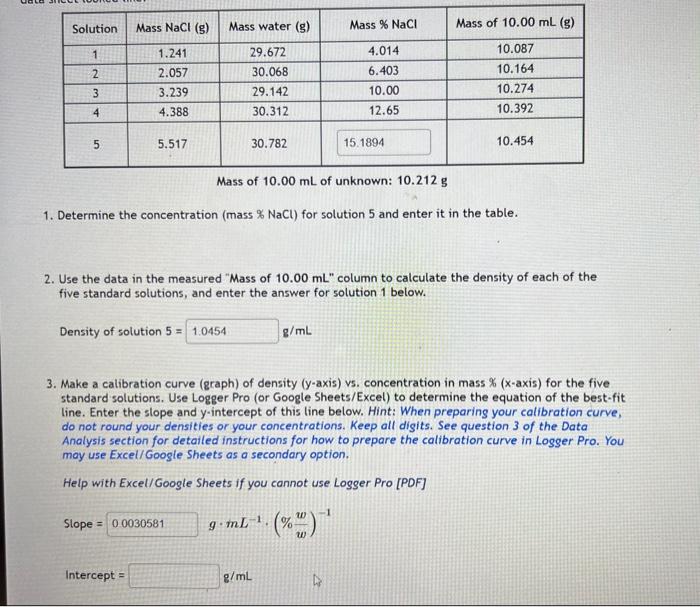
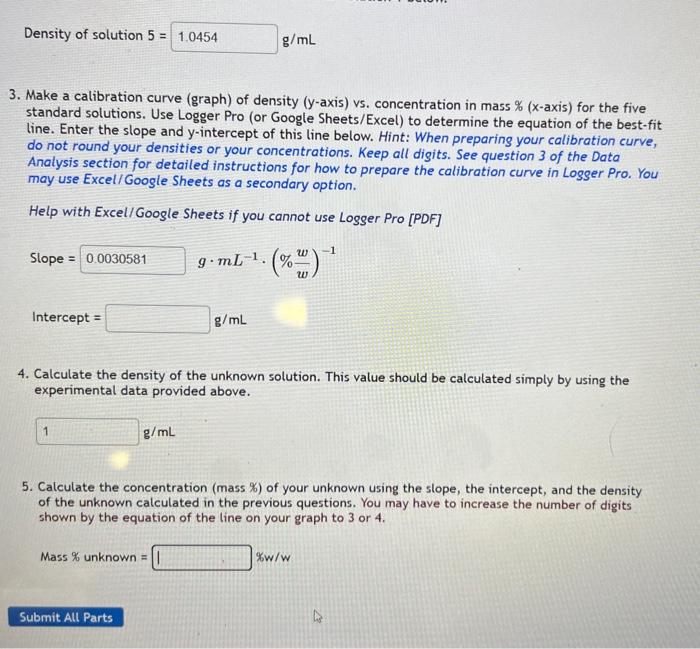
Mass of 10.00mL of unknown: 10.212g 1. Determine the concentration (mass %NaCl ) for solution 5 and enter it in the table. 2. Use the data in the measured "Mass of 10.00mL " column to calculate the density of each of the five standard solutions, and enter the answer for solution 1 below. Density of solution 5= g/mL 3. Make a calibration curve (graph) of density ( y-axis) vs. concentration in mass % ( x-axis) for the five standard solutions. Use Logger Pro (or Google Sheets/Excel) to determine the equation of the best-fit line. Enter the slope and y-intercept of this line below. Hint: When preparing your calibration curve, do not round your densities or your concentrations. Keep all digits. See question 3 of the Data Analysis section for detailed instructions for how to prepare the calibration curve in Logger Pro. You may use Excel/ Google Sheets as a secondary option. Help with Excel/Google Sheets if you cannot use Logger Pro [PDF] Slope = gmL1(%ww)1 Density of solution 5= g/mL 3. Make a calibration curve (graph) of density ( y-axis) vs. concentration in mass % ( x-axis) for the five standard solutions. Use Logger Pro (or Google Sheets/Excel) to determine the equation of the best-fit line. Enter the slope and y-intercept of this line below. Hint: When preparing your calibration curve, do not round your densities or your concentrations. Keep all digits. See question 3 of the Data Analysis section for detailed instructions for how to prepare the calibration curve in Logser Pro. You may use Excel/ Google Sheets as a secondary option. Help with Excel/ Google Sheets if you cannot use Logger Pro [PDF] Slope=Intercept=gmL1(%ww)1g/mL 4. Calculate the density of the unknown solution. This value should be calculated simply by using the experimental data provided above. 5. Calculate the concentration (mass \%) of your unknown using the slope, the intercept, and the density of the unknown calculated in the previous questions. You may have to increase the number of digits shown by the equation of the line on your graph to 3 or 4 . Mass % unknown = %w/w