Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass of acetylsal. acid : 1.5622 g Mass of acetlysal. acid per tablet: 325mg Molar mass: 180 Using all given info please solve #7, #8,
Mass of acetylsal. acid : 1.5622 g
Mass of acetlysal. acid per tablet: 325mg
Molar mass: 180
Using all given info please solve #7, #8, #9
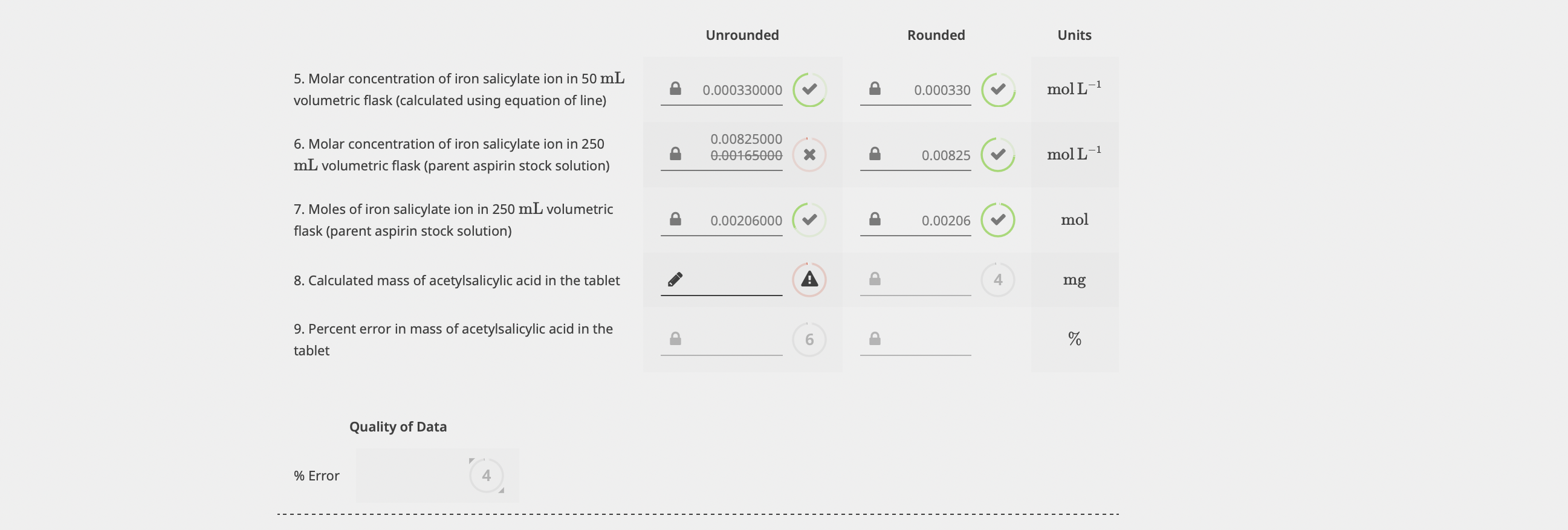
Unrounded Rounded Units 5. Molar concentration of iron salicylate ion in 50mL volumetric flask (calculated using equation of line) 0.000330000 0 0.000330 mol m1 6. Molar concentration of iron salicylate ion in 250 mL volumetric flask (parent aspirin stock solution) 0.008250000.00165000 x) 0.00825 molL1 7. Moles of iron salicylate ion in 250mL volumetric flask (parent aspirin stock solution) Q 0.00206000 a 0.00206 mol 8. Calculated mass of acetylsalicylic acid in the tablet 4mg 9. Percent error in mass of acetylsalicylic acid in the tablet ( 6) % Quality of Data \% Error 4Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


