Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Melting point measurements Table 1. Melting point of pure compounds Start melting temperature (C) Complete melted temperature (C) Pure benzoic acid 122 125 Pure
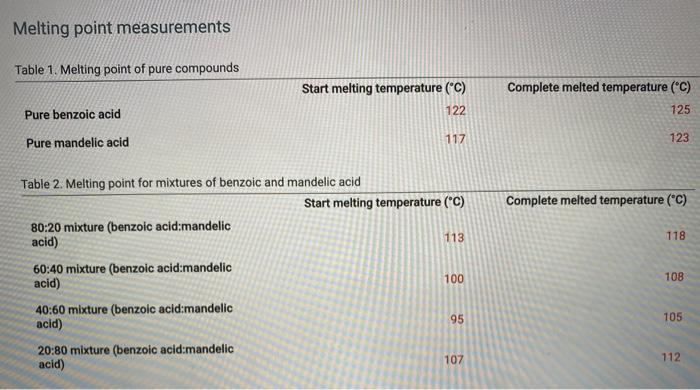
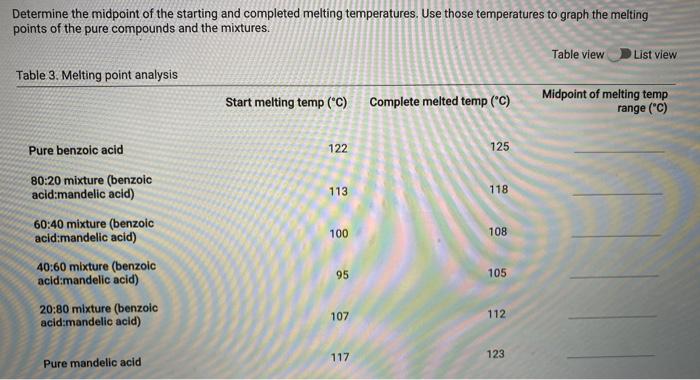

Melting point measurements Table 1. Melting point of pure compounds Start melting temperature ("C) Complete melted temperature ("C) Pure benzoic acid 122 125 Pure mandelic acid 117 123 Table 2. Melting point for mixtures of benzoic and mandelic acid Start melting temperature ("C) Complete melted temperature ("C) 80:20 mixture (benzoic acid:mandelic acid) 113 118 60:40 mixture (benzoic acid:mandelic acid) 100 108 40:60 mixture (benzoic acid:mandelic acid) 95 105 20:80 mixture (benzoic acid:mandelic acid) 107 112 Determine the midpoint of the starting and completed melting temperatures, Use those temperatures to graph the melting points of the pure compounds and the mixtures. Table view List view Table 3. Melting point analysis Start melting temp ("C) Complete melted temp ("C) Midpoint of melting temp range ("C) Pure benzoic acid 122 125 80:20 mixture (benzoic acid:mandelic acid) 113 118 60:40 mixture (benzoic acid:mandelic acid) 100 108 40:60 mixture (benzoic acid:mandelic acid) 95 105 20:80 mixture (benzoic acid:mandelic acid) 107 112 117 123 Pure mandelic acid please answer the following qustions by using the data that I attached. I need the answers Type not hand write please! 1/ do the garph 2/ what is the melting point of benzoic acid that you determined? 3/ what does this tell you about the purity of the compound? 4/ what was the eutectic temperature (temperature from the two lines of the best fit cross) for 5/ what was the composition of the mixture from the graph? 6/ where should you dispose of capillary tubes when you have finished determining melting p i forgot to mention ( you have to answer Table 3) melting point analysis
Step by Step Solution
★★★★★
3.57 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
answered this miptemp range pace benzoic ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


