Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methane enters an old furnace at a rate of 3 0 0 . 0 k g h . It is accompanied by 3 1 .
Methane enters an old furnace at a rate of It is accompanied by excess air containing mol water and the
remainder a : molar ratio of to Mixing of the combustion gases is very poor so that incomplete combustion is significant.
The combustion reactions proceed according to
Take the stoichiometric coefficients to have units of kmoles.
A significant amount of uncombusted methane leaves the furnace. The exhaust gas contains and on a dry
basis.Calculate the molar flow rate of each species in the feed.
Calculate the total mass flow rate into the furnace:
Check basis for composition of exhaust gas.
Try working the problem using molecular balances.
Draw and label a flow diagram and use appropriate balances to calculate the rate of change of the extents of reactions and
above. Take the stoichiometric coefficients in each reaction to have units of kmoles.
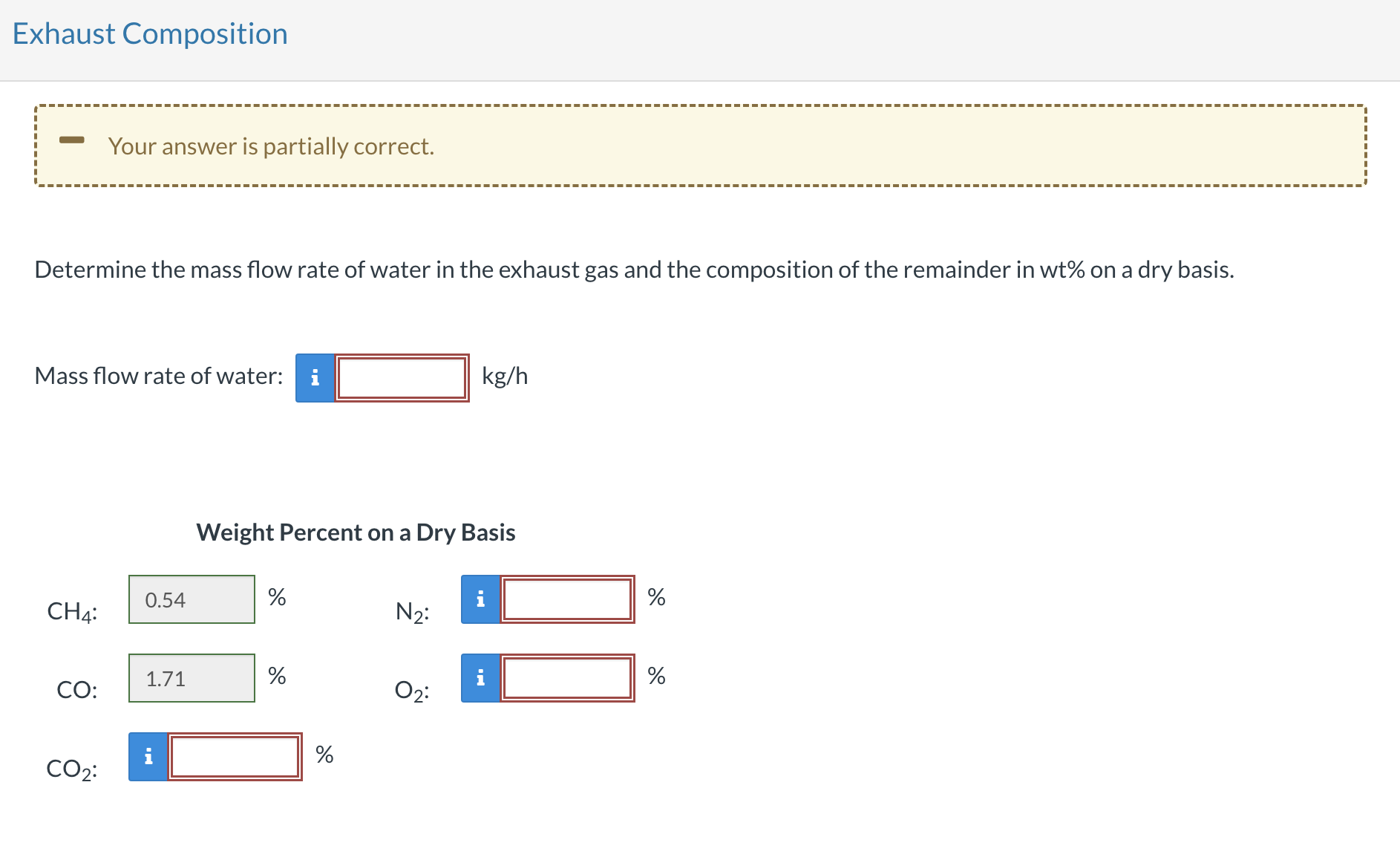
Determine the mass flow rate of water in the exhaust gas and the composition of the remainder in wt on a dry basis.
Mass flow rate of water:
Weight Percent on a Dry Basis
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


