Answered step by step
Verified Expert Solution
Question
1 Approved Answer
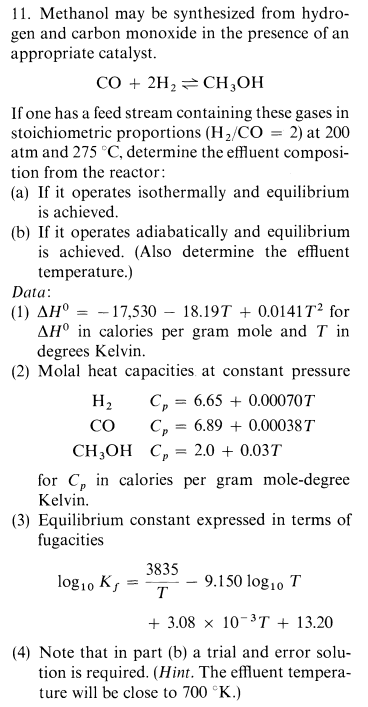
Methanol may be synthesized from hydro - gen and carbon monoxide in the presence of an appropriate catalyst. C O + 2 H 2 C
Methanol may be synthesized from hydro
gen and carbon monoxide in the presence of an
appropriate catalyst.
If one has a feed stream containing these gases in
stoichiometric proportions at
atm and determine the effluent composi
tion from the reactor:
a If it operates isothermally and equilibrium
is achieved.
b If it operates adiabatically and equilibrium
is achieved. Also determine the effluent
temperature.
Data:
for
in calories per gram mole and in
degrees Kelvin.
Molal heat capacities at constant pressure
for in calories per gram moledegree
Kelvin.
Equilibrium constant expressed in terms of
fugacities
Note that in part b a trial and error solu
tion is required. Hint The effluent tempera
ture will be close to
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


