Answered step by step
Verified Expert Solution
Question
1 Approved Answer
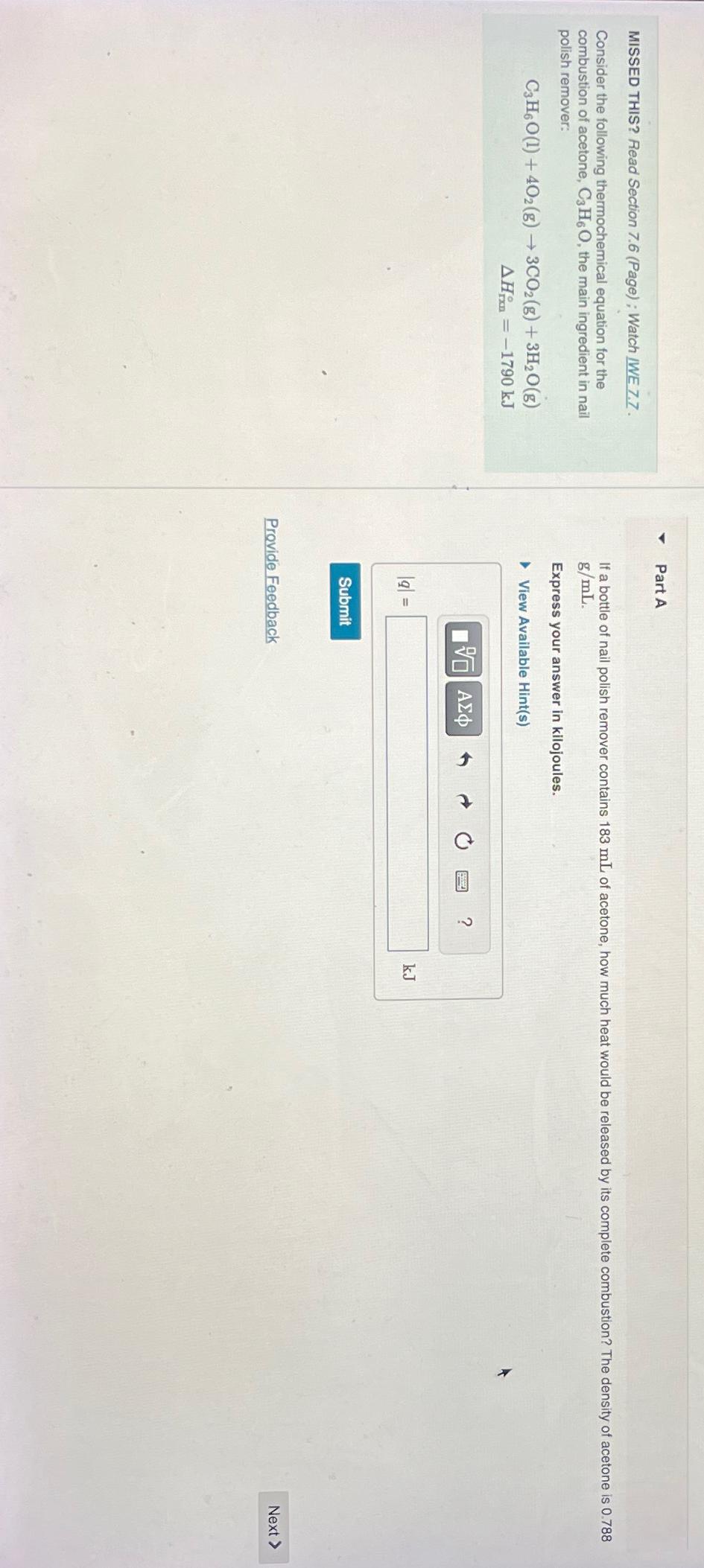
MISSED THIS? Read Section 7 . 6 ( Page ) ; Watch IWE 7 . 7 . Consider the following thermochemical equation for the combustion
MISSED THIS? Read Section Page ; Watch IWE
Consider the following thermochemical equation for the combustion of acetone, the main ingredient in nail polish remover:
Part A
If a bottle of nail polish remover contains of acetone, how much heat would be released by its complete combustion? The density of acetone is
Express your answer in kilojoules.
View Available Hints
:
:
Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


