
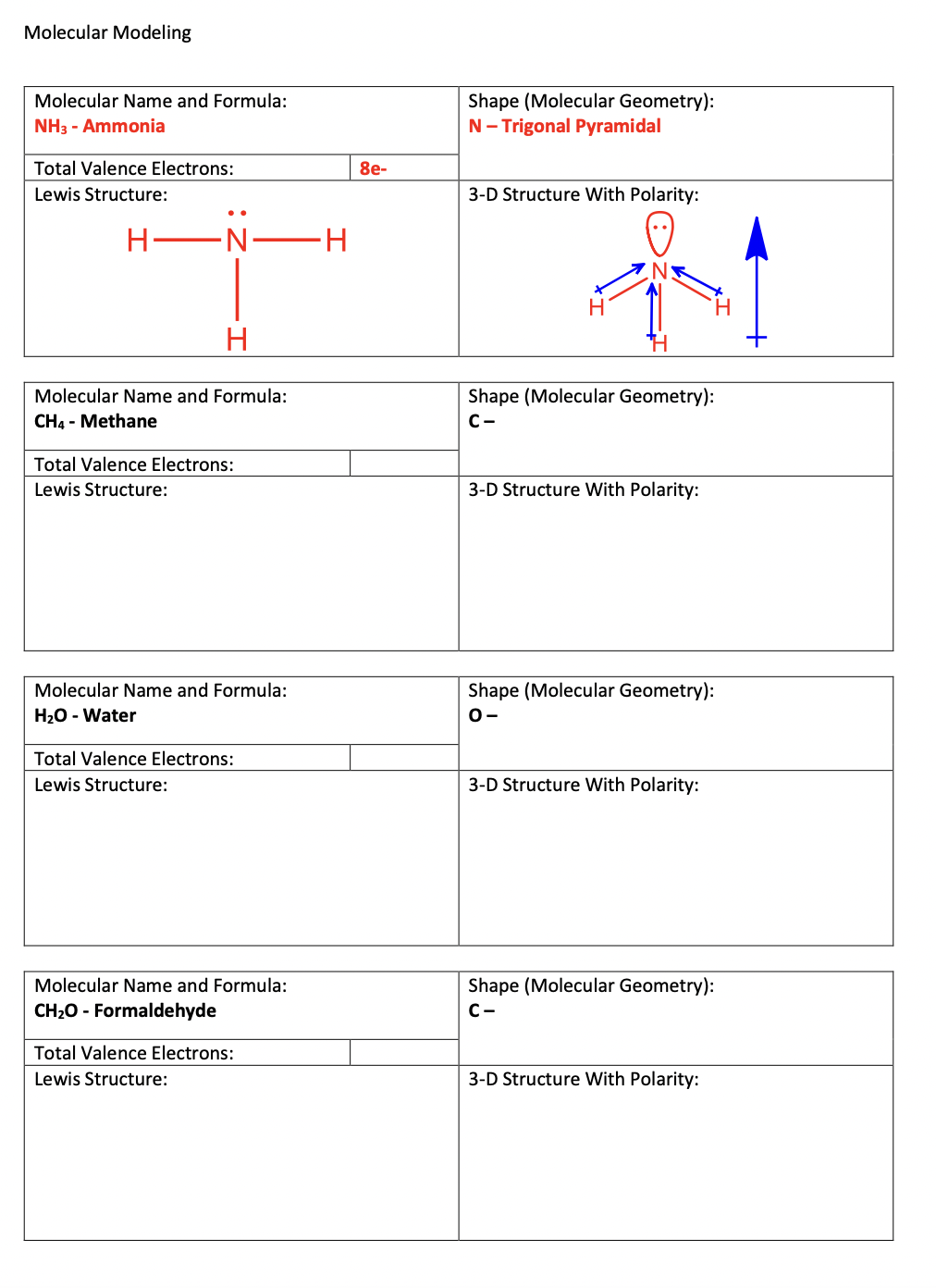
Molecular Modeling The following worksheet is designed to teach you to draw simple molecules, interpret the geometry of their central atom(s), and assign an overall polarity to the molecule. To complete this exercise, you will need a printed copy of this document, a periodic table with electronegativity values, and a computer with access to v For each molecule that you will draw, you will see a table like the one below: To draw any structure, follow these steps. 1. For the compound given in box A, count the total number of valence electrons across all atoms. Enter the total into box B. 2. Predict the framework of the Lewis structure. Connect all atoms together into the framework with a single bond. Draw this structure in box C. - The central atom is typically listed first and is usually the atom that prefers the most bonds. Typical bond count: C4,N3,O/S2,H/Cl1 - Larger molecules will have multiple "central" atoms. In this worksheet, larger molecules have a frame already provided for you - Hydrogens only accept 1 bond and thus should not be connected to each other 3. For each bond used in the framework, subtract 2 electrons from the valence electron total. Use any remaining electrons as lone pairs on atoms that do not have a filled octet. 4. After assigning all remaining electrons, if any atoms have an unfilled octet, reassign lone pairs as double bonds or triple bonds, as necessary. - If you cannot get a structure to work, try rearranging your framework and starting again. 5. Once you have a finished Lewis structure, recreate your structure a J. - Draw your Lewis structure in the program window. Note that it will not show any lone pairs on your drawn Lewis structure. - Select "2D to 3D" to convert it into a molecular model - Based on the model, assign each central atom a molecular geometry according to VSEPR theory. Record it in box D. - In the "Jmol" menu, select "Bond Dipoles". This will compare the electronegativities of each atom in every bond and draw an arrow towards the more electronegative atom. - In the "Jmol" menu, select "Overall Dipole". This will sum all the individual bond dipoles together and create an overall polarity. If the molecule has no overall polarity, no arrow will appear. - Draw a representation of the 3D model and show the overall dipole arrow (if any) in box E. If the molecule has no overall dipole, write that the molecule is Non-Polar. Molecular Modeling








