Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The wave function can be factored into the product of three independent functions (0,y,r) = 0(0)$(4)R(r). The square of the so-called radial function, R2(r),
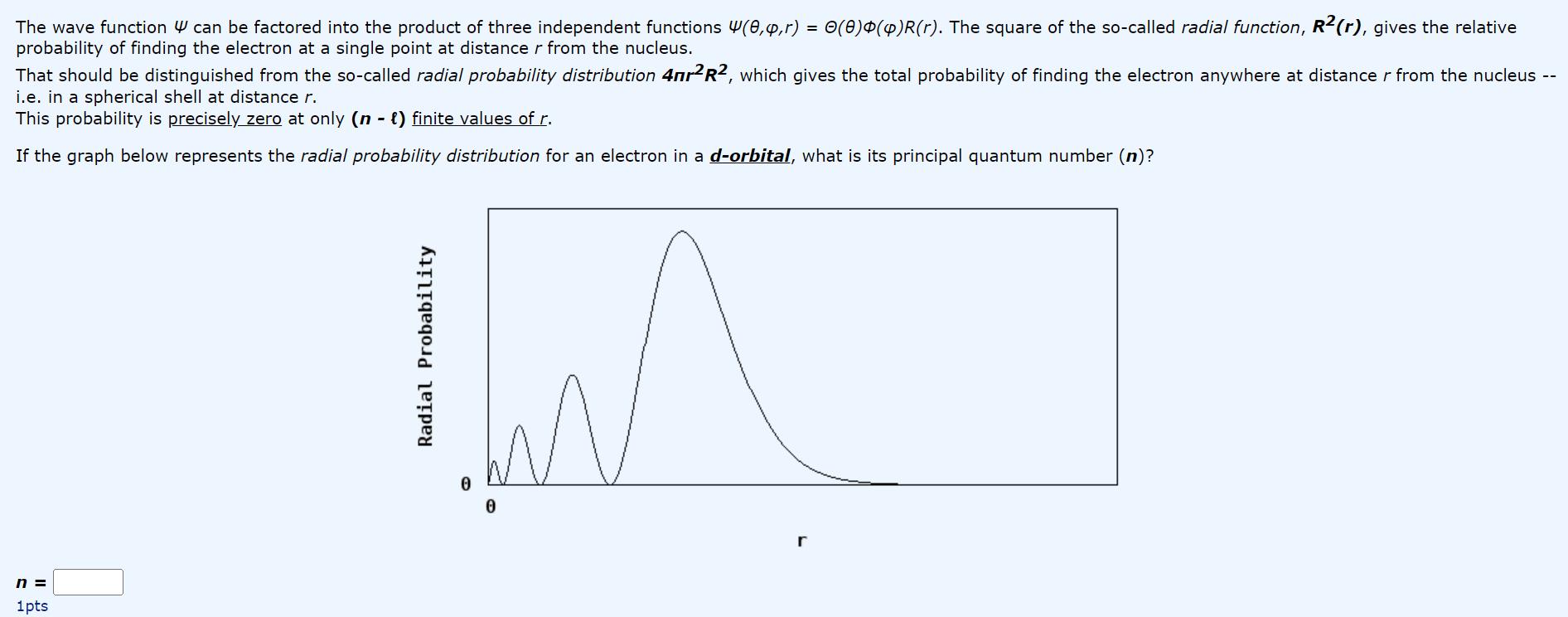
The wave function can be factored into the product of three independent functions (0,y,r) = 0(0)$(4)R(r). The square of the so-called radial function, R2(r), gives the relative probability of finding the electron at a single point at distance r from the nucleus. That should be distinguished from the so-called radial probability distribution 4nrR2, which gives the total probability of finding the electron anywhere at distance r from the nucleus -- i.e. in a spherical shell at distance r. This probability is precisely zero at only (n - ) finite values of r. If the graph below represents the radial probability distribution for an electron in a d-orbital, what is its principal quantum number (n)? n = 1pts Radial Probability 0 0 r
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The principal quantum number n is one of the quantum numbers that describes the energy levels and wa...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


