Question
My answer is correct but I need more detail about my answer, so watch the professor's comment and write the solution in more detail. I
My answer is correct but I need more detail about my answer, so watch the professor's comment and write the solution in more detail. I attach the question and the professor's comment. I attach my solution and comment. ***Don't use a calculator.
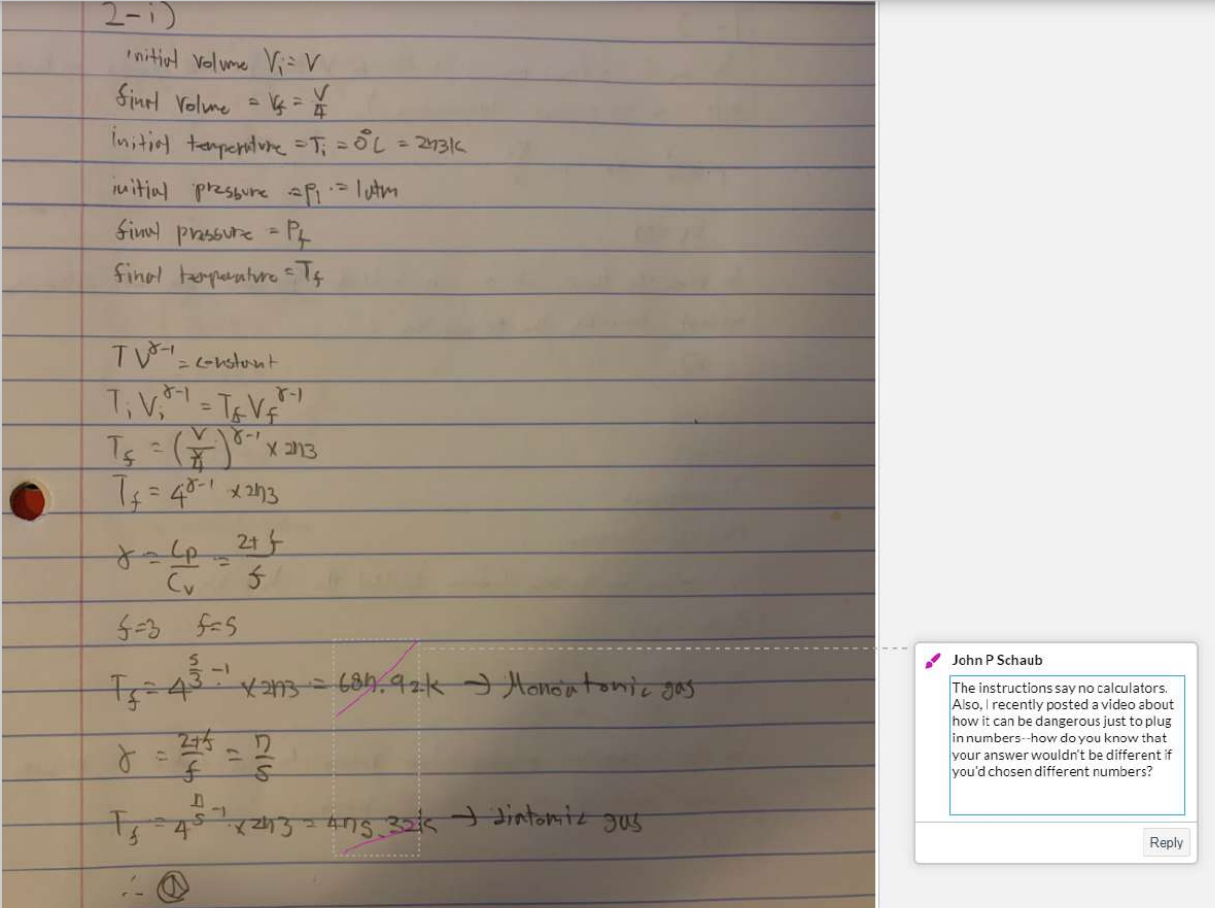
2. Consider two cylinders of gas identical in all respects except that one contains diatomic gas and the other a monoatomic gas. Both cylinders initially contain the same volume of gas at 0C and 1 atm of pressure and are closed by a movable piston at one end. Both gases are now compressed adiabatically to one-fourth their original volume.
(i) Which gas will show the smaller temperature increase? (Hint: Think about degrees of freedom.)
a. the diatomic gas
b. the monoatomic gas
c. Neither; both will show the same increase.
d. It is impossible to tell from the information given.
(ii) Which gas will show the greater pressure increase?
a. the diatomic gas
b. the monoatomic gas
c. Neither; both will show the same increase.
d. It is impossible to tell from the information given
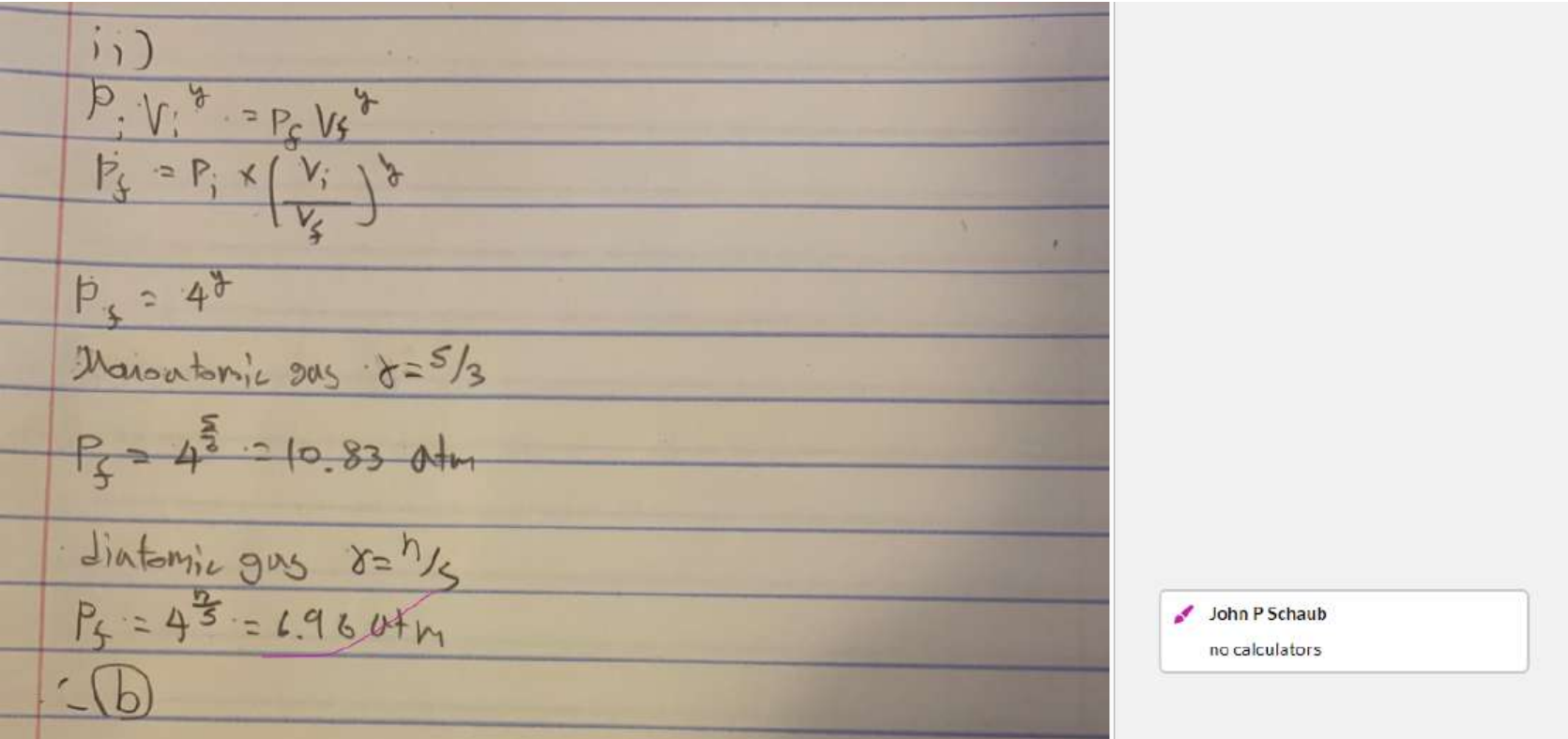

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


