Answered step by step
Verified Expert Solution
Question
1 Approved Answer
My graph and data: Use the data from the table to plot a titration curve where pH is on y-axis, and NaOH volume is on

My graph and data:
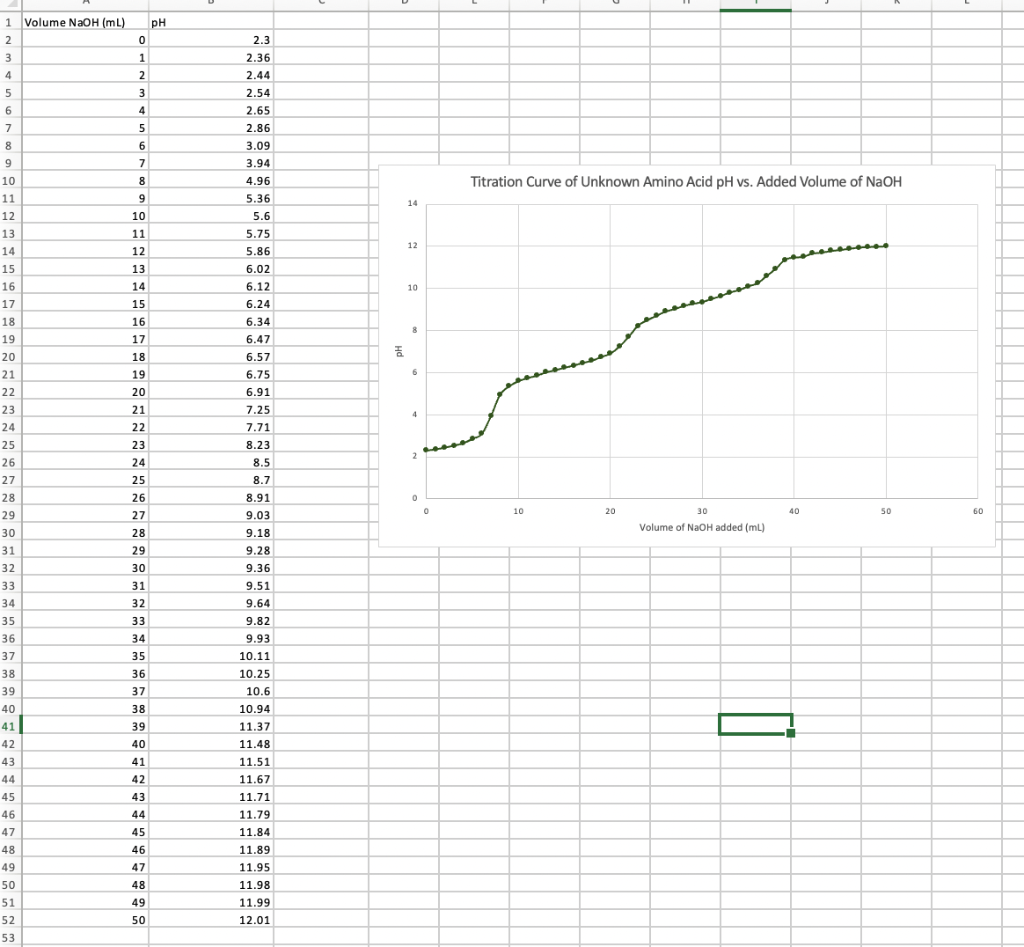
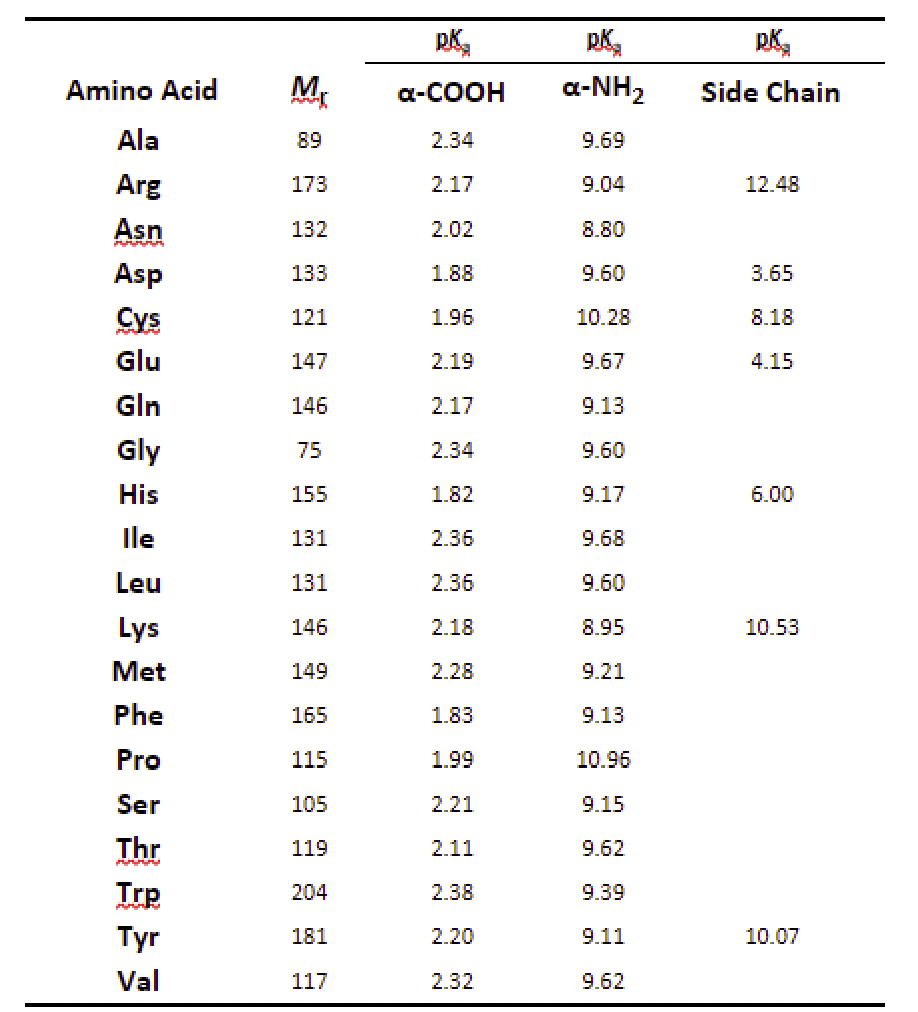
Use the data from the table to plot a titration curve where pH is on y-axis, and NaOH volume is on x-axis. The titration curve must have labeled axes, equivalence point(s), and 1/2 equivalence point(s). Compare the pKa value(s) you have obtained from the titration curve with known pKa values (Appendix B) to identify what the unknown amino acid is. \begin{tabular}{ccccc} \hline & & PKr & PKrr & DKrr \\ \cline { 3 - 5 } Amino Acid & MK & -COOH & NH2 & Side Chain \\ Ala & 89 & 2.34 & 9.69 & \\ Arg & 173 & 2.17 & 9.04 & 12.48 \\ Asn & 132 & 2.02 & 8.80 & \\ Asp & 133 & 1.88 & 9.60 & 3.65 \\ Cys & 121 & 1.96 & 10.28 & 8.18 \\ Glu & 147 & 2.19 & 9.67 & 4.15 \\ Gln & 146 & 2.17 & 9.13 & \\ Gly & 75 & 2.34 & 9.60 & \\ His & 155 & 1.82 & 9.17 & 6.00 \\ Ile & 131 & 2.36 & 9.68 & \\ Leu & 131 & 2.36 & 9.60 & \\ Lys & 146 & 2.18 & 8.95 & 10.53 \\ Met & 149 & 2.28 & 9.21 & \\ Phe & 165 & 1.83 & 9.13 & \\ Pro & 115 & 1.99 & 10.96 & \\ Ser & 105 & 2.21 & 9.15 & \\ Thr & 119 & 2.11 & 9.62 & \\ Trp & 204 & 2.38 & 9.39 & \\ Tyr & 181 & 2.20 & 9.11 & 10.07 \\ Val & 117 & 2.32 & 9.62 & \\ \hline \end{tabular} Titration Curve of Unknown Amino Acid pH vs. Added Volume of NaOH
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


