Question
1. Calculate the concentration, in grams of solute per 100 g of water, of a solution in which 7.462 g of solute dissolved in
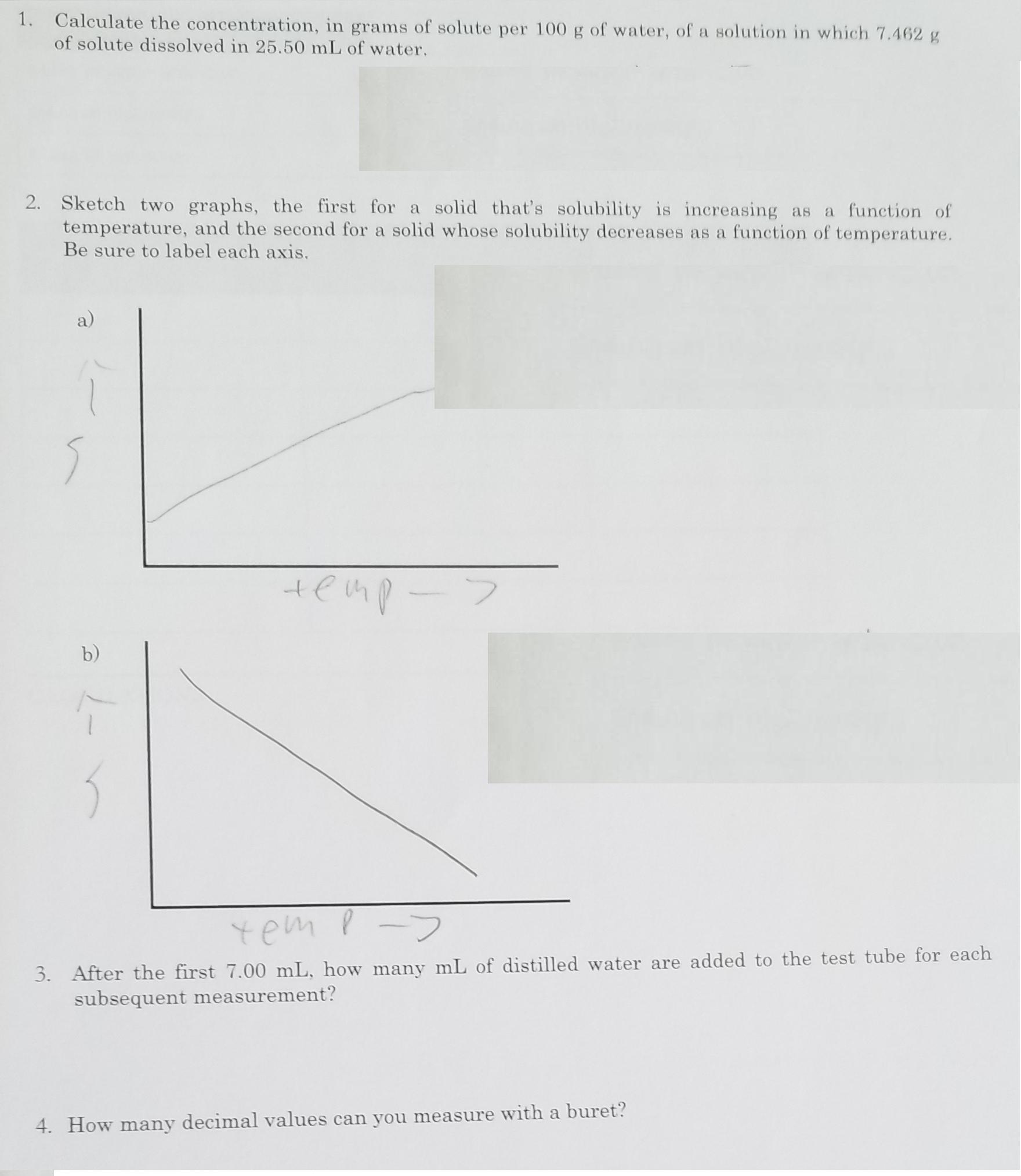
1. Calculate the concentration, in grams of solute per 100 g of water, of a solution in which 7.462 g of solute dissolved in 25.50 mL of water. 2. Sketch two graphs, the first for a solid that's solubility is increasing as a function of temperature, and the second for a solid whose solubility decreases as a function of temperature. Be sure to label each axis. a) b) yem p-> 3. After the first 7.00 mL, how many mL of distilled water are added to the test tube for each subsequent measurement? 4. How many decimal values can you measure with a buret?
Step by Step Solution
3.56 Rating (191 Votes )
There are 3 Steps involved in it
Step: 1
B 1 Given Mass of solute 7462 g Volume of solution 2550 ml To find Concentration in grams of solute ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Analytical Chemistry
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
9th edition
495558281, 978-0495558286
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



