Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Name Date Using the Thermodynamics table below solve the next equations. 1. 4HCN(1) + 502 (9) 2H2O(g) + 4CO2 (g) + 2N2 (g) Temp:30 C/

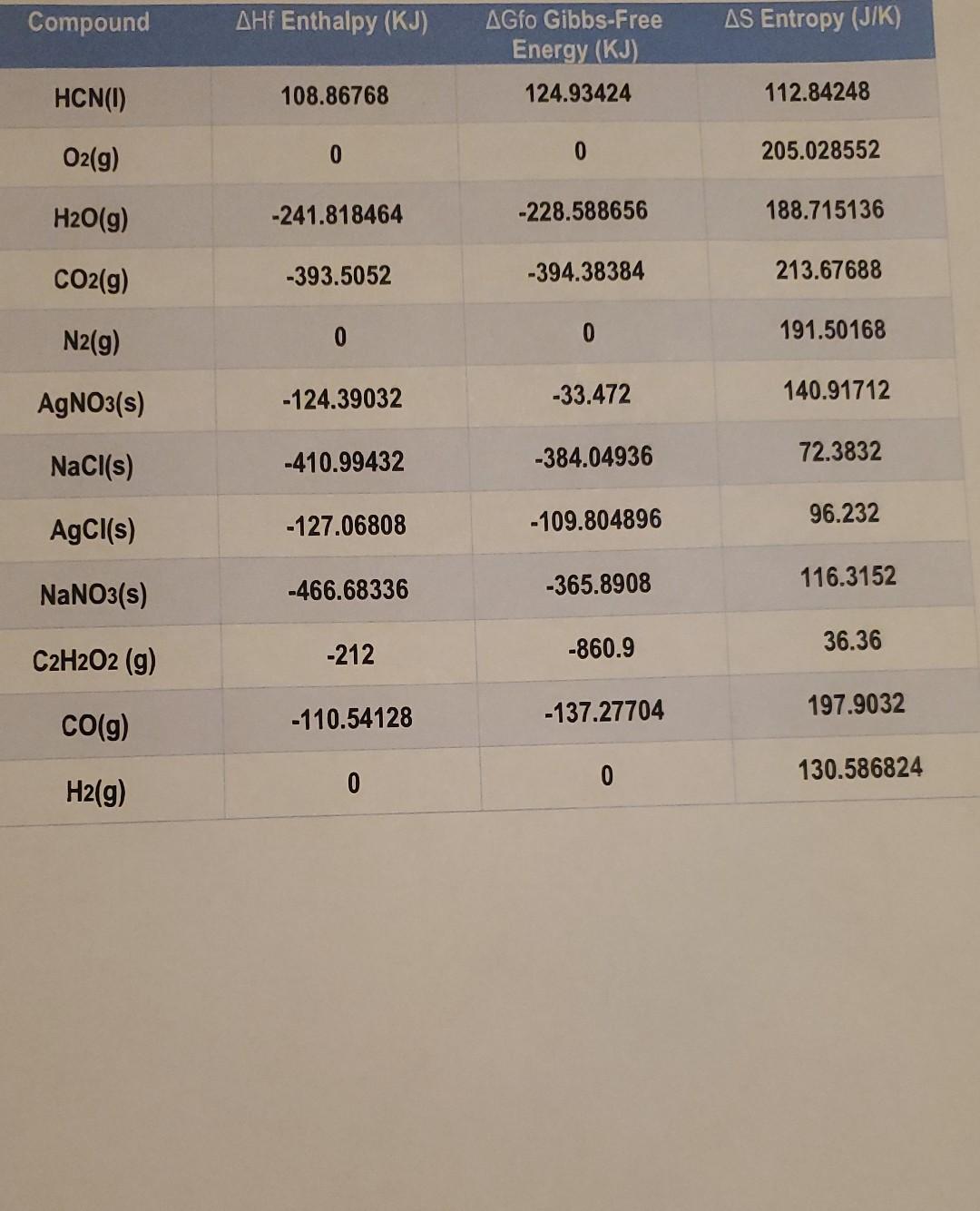
Name Date Using the Thermodynamics table below solve the next equations. 1. 4HCN(1) + 502 (9) 2H2O(g) + 4CO2 (g) + 2N2 (g) Temp:30 C/ Find AG using A G = AH-TAS 2. AgNO3(s) + NaCl(s) AgCl(s) + NaNO3 (s) Find AGO, Using AG = E AGf(products), AGf (reactants) 1 3. C2H2O2(g) 200(g) + H2(g) Find AHO, Using AH = E AHf(products), E AHf(reactants) > 1 Compound AHf Enthalpy (KJ) AS Entropy (J/K) AGfo Gibbs-Free Energy (KJ) 124.93424 HCN) 108.86768 112.84248 O2(g) 0 0 205.028552 H2O(g) -241.818464 -228.588656 188.715136 CO2(g) -393.5052 -394.38384 213.67688 N2(9) 0 0 191.50168 AgNO3(s) -124.39032 -33.472 140.91712 NaCl(s) -410.99432 -384.04936 72.3832 AgCl(s) -127.06808 -109.804896 96.232 NaNO3(s) -466.68336 -365.8908 116.3152 36.36 C2H2O2 (g) -212 -860.9 -137.27704 197.9032 CO(g) -110.54128 0 130.586824 0 H2(g)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


