Question
N-butyl acetate is a synthetic flavoring with a sweet banana or apple aroma that is commonly used in the production of cakes, candies, or ice
N-butyl acetate is a synthetic flavoring with a sweet banana or apple aroma that is commonly used in the production of cakes, candies, or ice cream. This compound is produced through an esterification reaction between n-butanol and ethanol. To maximize conversion, the molar ratio between n-butanol and ethanol was set in the range of 1:5 to 1:7.
N-butyl acetate is produced by the reactive distillation method, which combines the reaction process and separates the product and the rest of the reactants in 1 column.
N-butanol (1000 mol/hour) and ethanol with a molar ratio of 1:5 were reacted and assumed to have a 100% conversion reaction according to the following reaction: N-butanol + ethanol n-butyl acetate + H2O
The water in the reaction product has been separated completely, while the remaining ethanol and the product will be separated by distillation. The desired upper product specification has a 10%-mole concentration of n-butyl acetate, while the lower product contains 5%-mole ethanol. This mixture is fed to a saturated vapor state. calculate: a. Number of top and bottom products produced b. Minimum reflux value
c. The number of theoretical stages if using total reflux d. The number of real stages if the reflux value is set to 1.25 times the minimum reflux, and the tray efficiency is 75%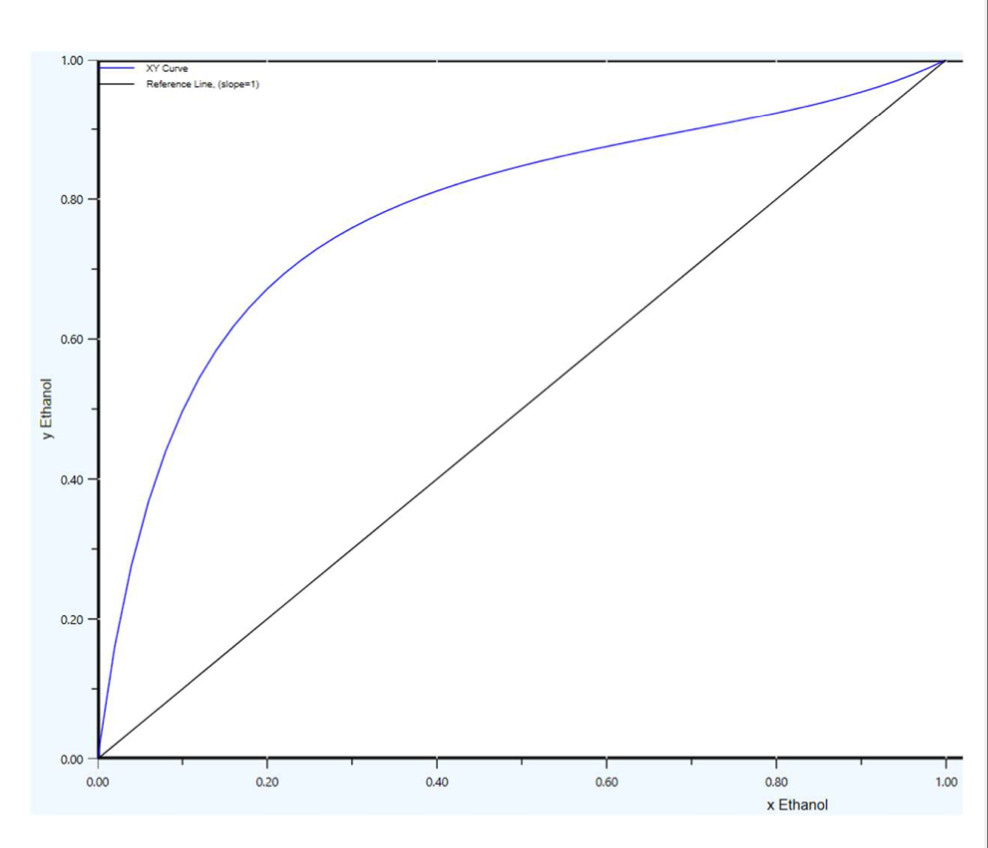
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


