Question
When Zinc chloride (ZnCl,) reacts with NH, solution, a white crystalline compound Zn(NH,), C, is produced. A student used the procedure in this module
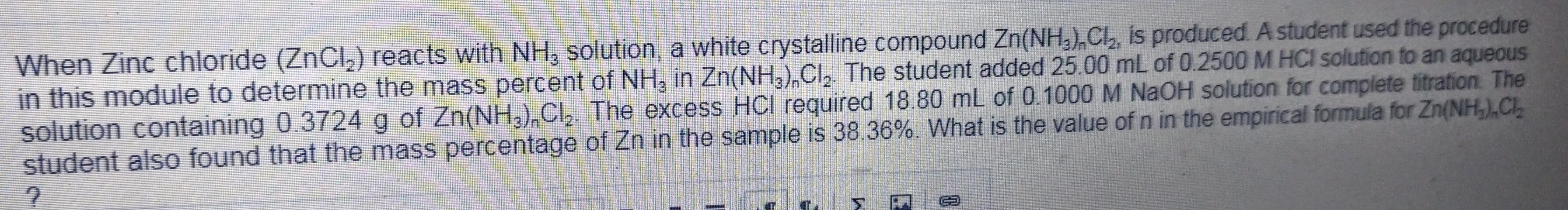
When Zinc chloride (ZnCl,) reacts with NH, solution, a white crystalline compound Zn(NH,), C, is produced. A student used the procedure in this module to determine the mass percent of NH, in Zn(NH,),CI, The student added 25.00 mL of 0.2500 M HCI solution to an aqueous solution containing 0.3724 g of Zn(NH,),Cl, The excess HCI required 18.80 mL of 0.1000 M NAOH solution for complete titration The student also found that the mass percentage of Zn in the sample is 38.36%. What is the value of n in the empirical formula for Zn(NH,),C
Step by Step Solution
3.46 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6366201776827_240374.pdf
180 KBs PDF File
6366201776827_240374.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


