Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need answers to both:) 7. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose when dissolved in water, ine molecular formula for
need answers to both:) 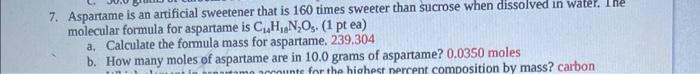

7. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose when dissolved in water, ine molecular formula for aspartame is C14H10N2O5. (1 pt ea) a. Calculate the formula mass for aspartame. 239.304 b. How many moles of aspartame are in 10.0 grams of aspartame? 0.0350 moles 8. Ascorbic acid, or vitamin C has the formula C6H6O6 and is an essential vitamin. It cannot be stored by the body and must be present in the diet. Ascorbic acid tablets are often taken as a dietary supplement. A typical tablet contains 500.0mg of vitamin C. (1 pt ea) a. What is the molecular weight (formula mass) of vitamin C? 176.124 grams b. How many moles of vitamin C does a typical tablet contain? 0.002839 moles c. What is the percent composition of oxygen in ascorbic acid? 55% or 54.5 d. What is the empirical formula for ascorbic acid? C3H4O3 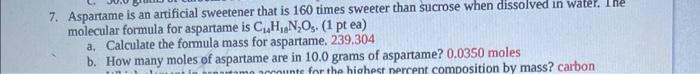

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


