Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help finding the theoretical yield. CHEM 334L - THEORETICAL YIELD PROBLEM SET NAMES: Determine the limiting reagent & calculate the theoretical yield of the
need help finding the theoretical yield. 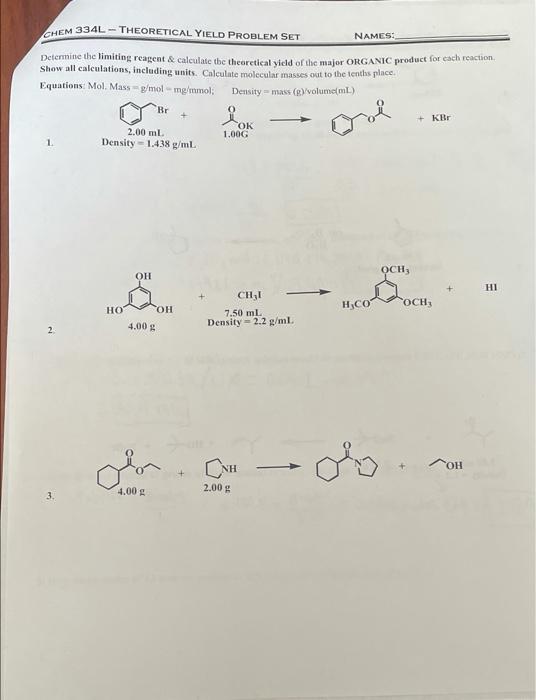
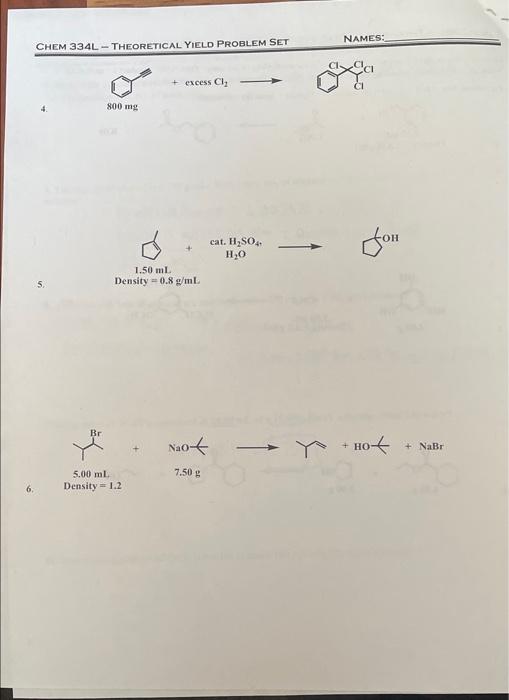
CHEM 334L - THEORETICAL YIELD PROBLEM SET NAMES: Determine the limiting reagent & calculate the theoretical yield of the major ORGANIC product for each reaction Show all calculations, including units. Calculate molecular masses out to the tenths place. Equations. Mol. Mass g/mol - mg/mmol; Density mass (g/volume ml) Br + KBr 2.00 ml 1.00G 1 Density - 1.438 g/mL. + OCH OH HI TOCH H.CO HO CH, 7.50 mL Density = 2.2 g/mL. 2 4.00 gim.Com - oby.com 3. 4.00 2.00 NAMES: CHEM 334L-THEORETICAL YIELD PROBLEM SET + excess Cl, 800 mg cat. H2SO4 H2O 1.50 mL Density -0.8 gml 5. Br Not + HO + NaBr 7.50 g 5.00 mL Density = 1.2 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


