Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with 7a-d 7. Use the ACSM metabolic equation (Table 4.1) for jogging to estimate the following: a. The gross VO2 in mlOz-kg min
need help with 7a-d 
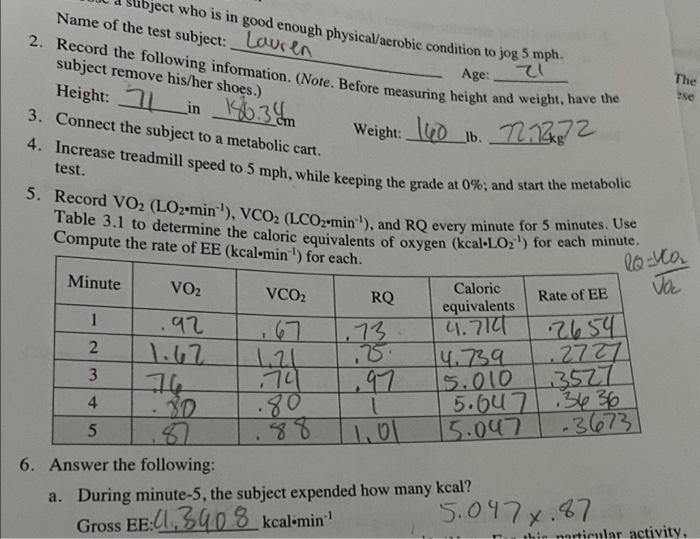
7. Use the ACSM metabolic equation (Table 4.1) for jogging to estimate the following: a. The gross VO2 in mlOz-kg min for jogging at 5 mph at a 0% grade. b. The net VO2 in mlO2-kg min for jogging at this workload. (Refer to Equation 4.8. and use 35 ml-kg-min' for resting VO2) c. The net EE in kcal-min' for jogging at this workload. (Hint. First, convert net VO2 expressed in relative terms to absolute terms.) d. The net EE (in total kcal) for jogging at this workload for 30 minutes. ject who is in good enough physical/aerobic condition to jog 5 mph. Name of the test subject: Louren 2. Record the following information. (Note. Before measuring height and weight, have the subject remove his/her shoes.) : 3. Connect the subject to a metabolic cart. 4. Increase treadmill speed to 5 mph, while keeping the grade at 0%, and start the metabolic test. Age: Height: 21 in in 186.34 The se Weight: 160 lb. 7212372 5. Record VO2 (LO2-min), VCO2 (LCO2-min), and RQ every minute for 5 minutes. Use Compute the rate of EE (kcal-min) for each. Minute VO2 VCO2 - 1 RQ-Co Caloric RQ Rate of EE Jo 1 equivalents 92 .73 4.712 2654 2 12 123 14.739 .277 3 79 97 5.010 13527 4 30 .80 5.6u7 .3636 5 8 88 1, 15.047 -3673 6. Answer the following: a. During minute-5, the subject expended how many kcal? Gross EE:[1,390 8 kcalimin 5.0478.87 hin narticular activity 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


