Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with d and e thanks CH3CH2CH2CH2OHcat3CH=CHCH3+H2O The rate law is rBu=(1+KBuPBu)2kPBu with k=0.054mol/gcathatm and KBu=0.32atm1. Pure butanol enters a thin-tubed, packedbed reactor at
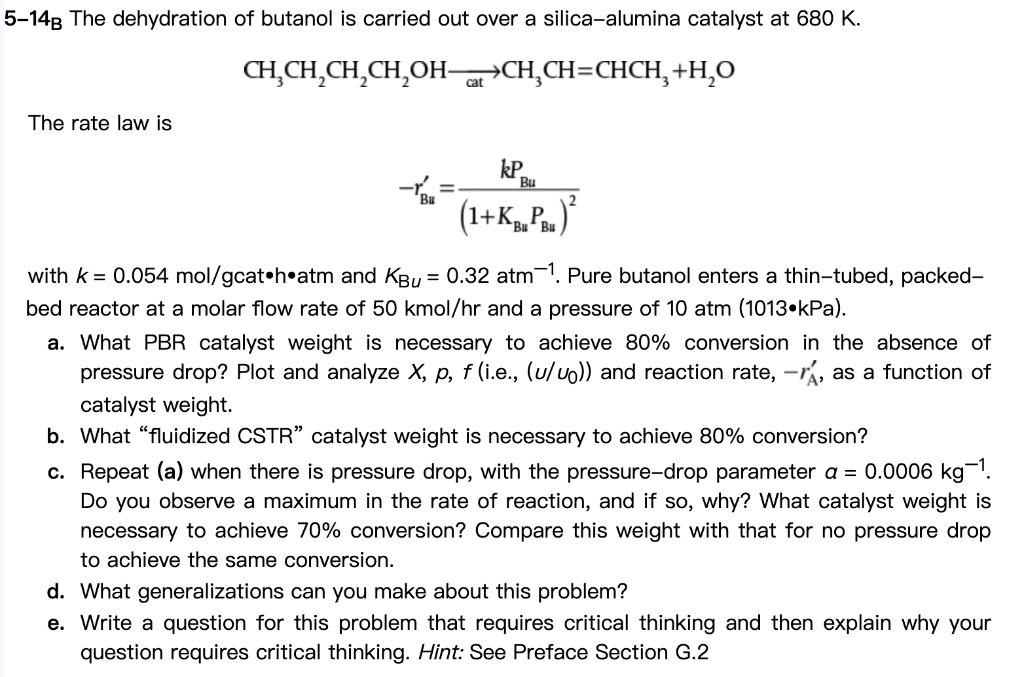
need help with d and e thanks
CH3CH2CH2CH2OHcat3CH=CHCH3+H2O The rate law is rBu=(1+KBuPBu)2kPBu with k=0.054mol/gcathatm and KBu=0.32atm1. Pure butanol enters a thin-tubed, packedbed reactor at a molar flow rate of 50kmol/hr and a pressure of 10atm(1013kPa). a. What PBR catalyst weight is necessary to achieve 80% conversion in the absence of pressure drop? Plot and analyze X,p,f( i.e., (u/u0)) and reaction rate, rA, as a function of catalyst weight. b. What "fluidized CSTR" catalyst weight is necessary to achieve 80% conversion? c. Repeat (a) when there is pressure drop, with the pressure-drop parameter a=0.0006kg1. Do you observe a maximum in the rate of reaction, and if so, why? What catalyst weight is necessary to achieve 70% conversion? Compare this weight with that for no pressure drop to achieve the same conversion. d. What generalizations can you make about this problem? e. Write a question for this problem that requires critical thinking and then explain why your question requires critical thinking. Hint: See Preface Section G.2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


