Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with molarity questions!! T1=(molaritysolution)(1.86C/m)molaritysolution(Trial1)=molaritysolution(Trial2)=mmMolaritysolution=molethylenesiycol/(kgwater)molethyleneglycol(Trial1)=molethylenegiycol(Trial2)=molarmassethyleneglycol(Trial1)=Averagemolarmassethyleneslywol=g/molg/molg/mol The actual molar mass of ethylene glycol is 62.07g/mol. Calculate the % error for your average molar mass: %
need help with molarity questions!! 
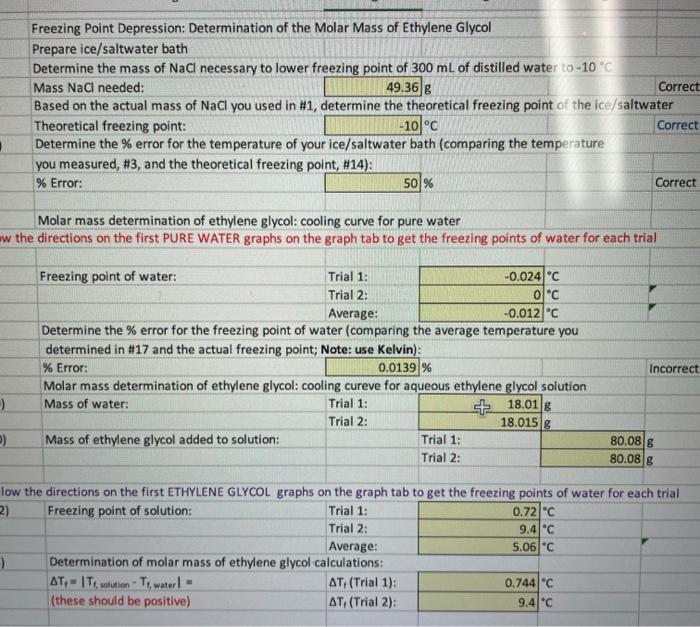
T1=(molaritysolution)(1.86C/m)molaritysolution(Trial1)=molaritysolution(Trial2)=mmMolaritysolution=molethylenesiycol/(kgwater)molethyleneglycol(Trial1)=molethylenegiycol(Trial2)=molarmassethyleneglycol(Trial1)=Averagemolarmassethyleneslywol=g/molg/molg/mol The actual molar mass of ethylene glycol is 62.07g/mol. Calculate the \% error for your average molar mass: % Error: % Incorrec Freezing Point Depression: Determination of the Molar Mass of Ethylene Glycol Prepare ice/saltwater bath Determine the mass of NaCl necessary to lower freezing point of 300mL of distilled water to 10C Mass NaCl needed: g Based on the actual mass of NaCl you used in #1, determine the theoretical freezing point of the ice/saltwater Theoretical freezing point: C Correct Determine the % error for the temperature of your ice/saltwater bath (comparing the temperature you measured, \#3, and the theoretical freezing point, \#14): \% Error: % Correct Molar mass determination of ethylene glycol: cooling curve for pure water the directions on the first PURE WATER graphs on the graph tab to get the freezing points of water for each trial Determine the \% error for the freezing point of water (comparing the average temperature you determined in \#17 and the actual freezing point; Note: use Kelvin): \% Error: % Molar mass determination of ethvlene elvcol: cooline cureve for anuenus ethvlene olvrol solution low the directions on the first ETHYLENE GLYCOL graphs on the graph tab to get the freezing points of water for each trial 2) \begin{tabular}{|l|l|r|r|} \hline Freezing point of solution: & Trial 1: & 0.72 \\ & Trial 2: & 9.4 & C \\ & Average: & 5.06 & C \\ \hline natarminatian af malar mare af athidana alunal raleulatinne. \end{tabular} 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


