Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need Help with Question 10-15 If you have 5.71024 atoms of sodium, how many moles is that? Your Answer: Answer Question 11 (0.25 points) What
Need Help with Question 10-15 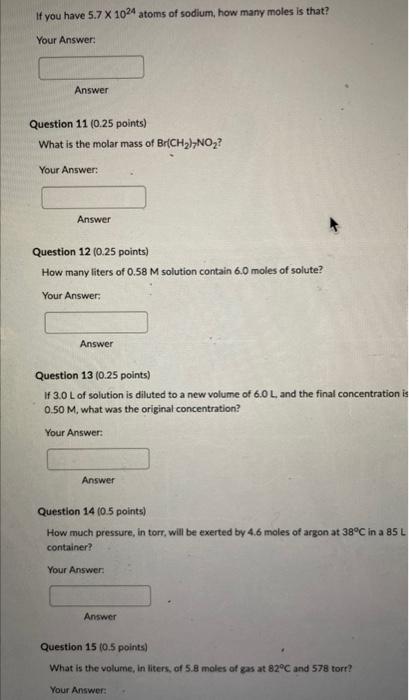
If you have 5.71024 atoms of sodium, how many moles is that? Your Answer: Answer Question 11 (0.25 points) What is the molar mass of Br(CH2)7NO2 ? Your Answer: Answer Question 12 (0.25 points) How many liters of 0.58M solution contain 6.0 moles of solute? Your Answer: Answer Question 13(0.25 points) If 3.0L of solution is diluted to a new volume of 6.0L, and the final concentration is 0.50M, what was the original concentration? Your Answer: Answer Question 14 (0.5 points) How much pressure, in torr, will be exerted by 4.6 moles of argon at 38C in a 85L container? Your Answer Answer Question 15 (0.5 points) What is the volume, in liters, of 5.8 moles of gas at 82C and 578 torr? Your 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


